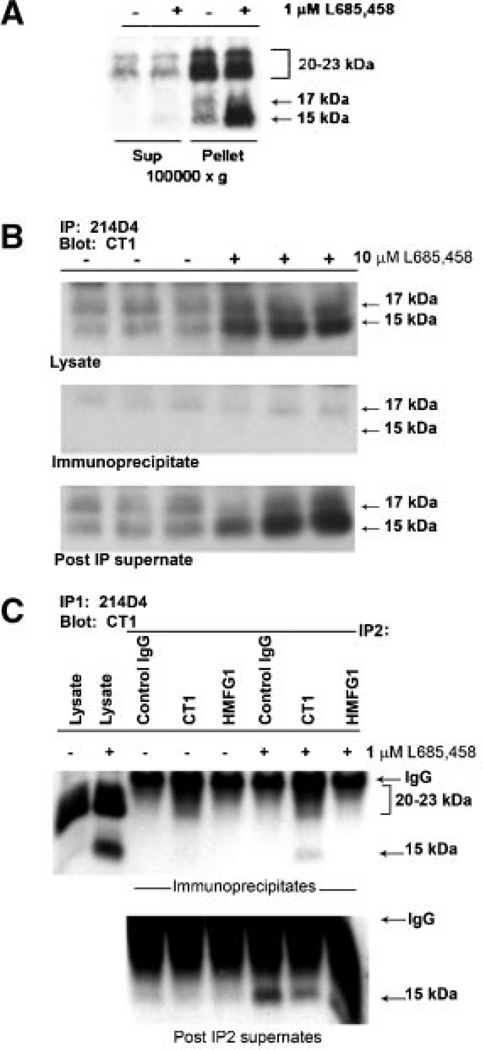
Fig. 3.
The MUC1 CTF15 is membrane associated but is not a component of the MUC1 “metabolic complex”. Panel A: Cytokine-stimulated HES cells were treated without or with 1 µM L685,458 for 54 h, homogenized and subjected to subcellular fractionation as described in “Experimental Procedures Section.” Resulting supernatants (Sup) and pellets (Pellet) were analyzed by SDS-PAGE and Western blotting with CT1 antibody as described in “Experimental Procedures Section.” MUC1 CTF15 as well as the 17 and 20–23 kDa components of the metabolic complex were associated with the 100,000g pellet. CTF15 was greatly enhanced in pellet fractions of cells treated with the γ-secretase inhibitor, L685,458. Equivalent proportions of each fraction were analyzed from both inhibitor treated and untreated cells. Panel B: Triplicate lysates from cytokine-stimulated HES cells treated without (−) or with (+) 10µM L685,458 were immunoprecipitated with MUC1 ectodomain antibody 214D4 and analyzed by SDS-PAGE and Western blotting with CT1 antibody. Only the region of the blot containing CTF17 and CTF15 in starting lysates (upper panel), immunoprecipitates (middle panel) or material not recognized by 214D4 (lower panel) is shown. Immunoblotting with CT1 antibody detected only the C-terminal MUC1 subunit (20–23 kDa doublet and CTF17) of the metabolic complex in the immunoprecipitate while the CTF15 remained in the post-IP supernatants. Panel C: To exclude the possibility that CTF15 might be associated with metabolic complex containing a glycoform not recognized by 214D4, ectodomain material in a post–214D4–immunoprecipitation supernatant subsequently was immunoprecipitated with control Ig (control), CT-1, or HMFG1 antibody. Immunoprecipitates (top panel) or IP supernatants (bottom panel) were analyzed by Western blotting with CT1 antibody. CTF15 was not present in HMFG1 immunoprecipitates. L, whole cell lysate.