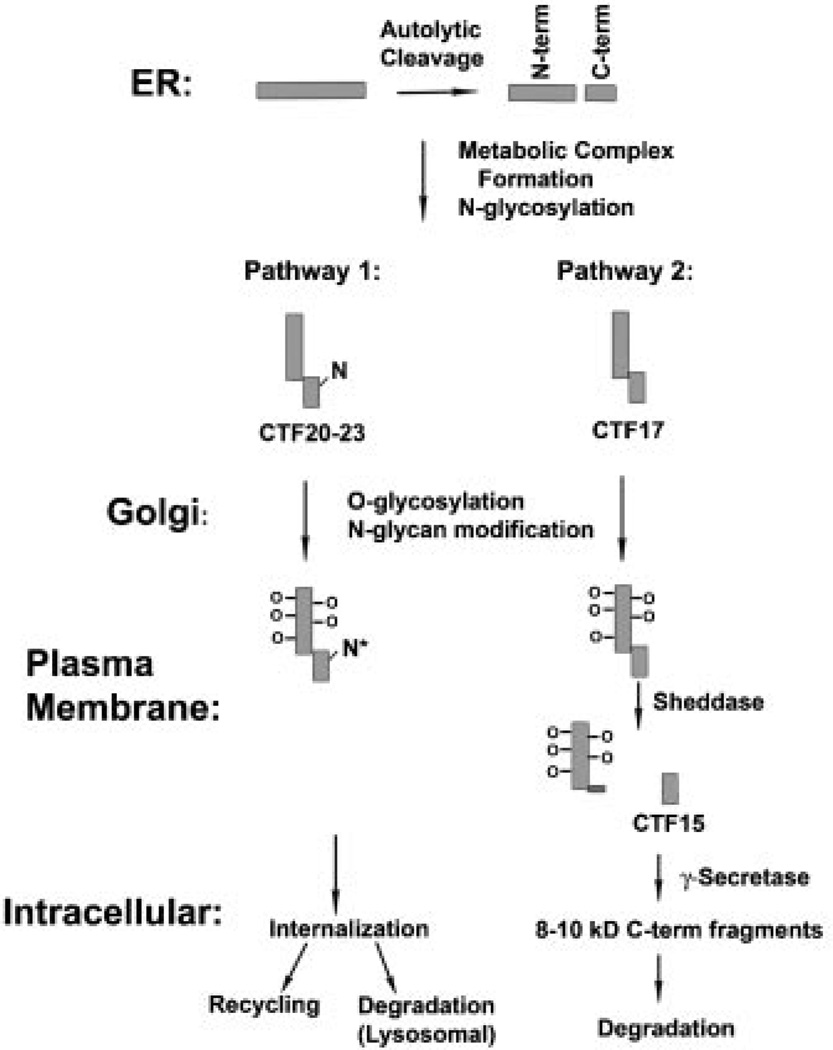
Fig. 8.
A model of MUC1 processing. The model summarizes MUC1 processing pathways from a number of studies discussed in the text. Only the contribution of N-glycosylation to the variety of C-terminal forms detected when MUC1F (full length MUC1 precursor) is overexpressed in normal cells is considered in this model. N-glycosylation sites are indicated by an N on the C-terminal subunits. Within the endoplasmic reticulum (ER), MUC1F is translated as a single polypeptide which folds to a conformation inducing an autolytic cleavage within the SEA module to produce N- and C-terminal subunits (N-term and C-term). The resulting subunits remain stably associated as a heterodimer (metabolic complex) and undergo N-glycosylation. There are five potential N-glycosylation sites in human MUC1: four within the N-terminal subunit and one within the C-terminal subunit. For simplicity, only the latter site is indicated on the figure and the N-glycosylated forms of this C-terminal subunit are labeled CTF20–23 in the figure and the text. Forms of the C-terminal subunit lacking N-glycosylation are labeled CTF17 in the figure and the text. MUC1 heterodimers transit the Golgi complex in which extensive O-glycosylation of the tandem repeat region occurs (indicated by O – in the figure) as well as additional modification of the N-glycan (N*). The present data indicates that upon arrival at the plasma membrane, the subset of heterodimers lacking N-glycans on the C-terminal fragment are substrates for sheddases that generate CTF15. CTF15 is a substrate for γ-secretase and is cleaved within the transmembrane domain at one or more sites. The C-terminal products of γ-secretase generally were undetectable and, therefore, presumed to be rapidly degraded; however, we detected trace amounts of 8–10 kDa C-terminal fragments in our studies which presumably are the products of γ-secretase destined for rapid degradation. Heterodimers containing N-glycosylated C-terminal subunits appear to be processed by an alternate pathway, possibly involving internalization, recycling, or lysosomal degradation.