Abstract
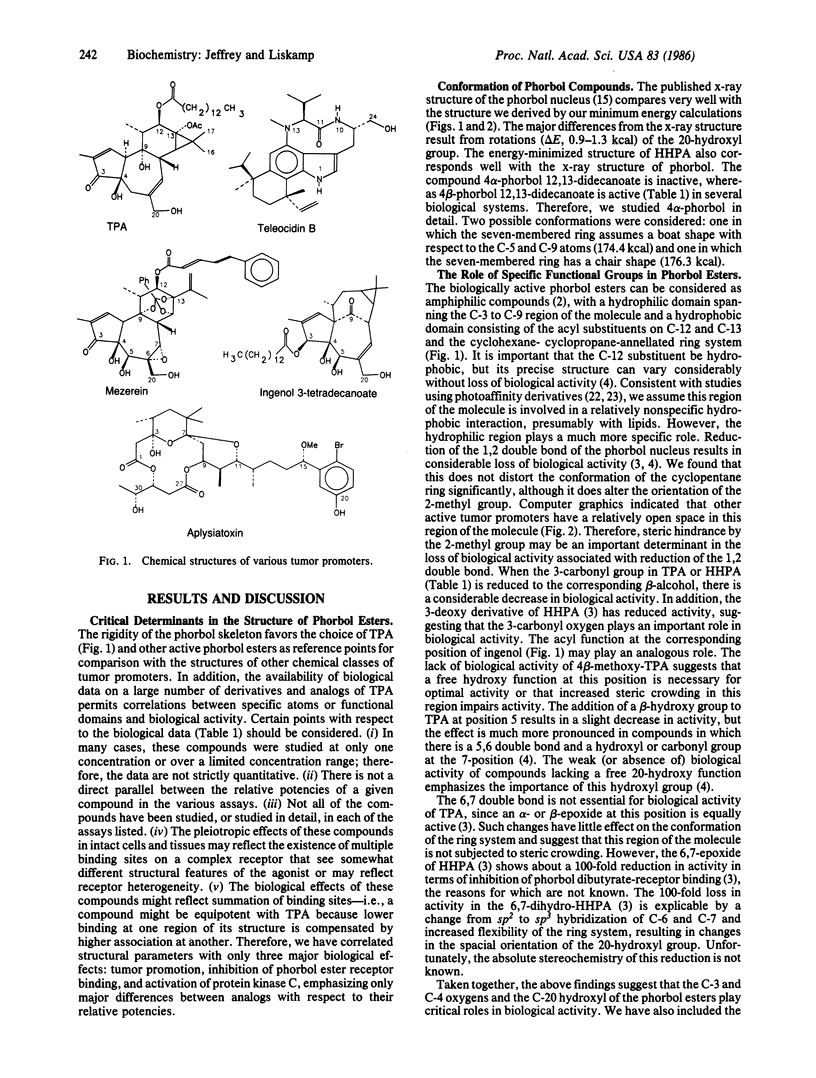
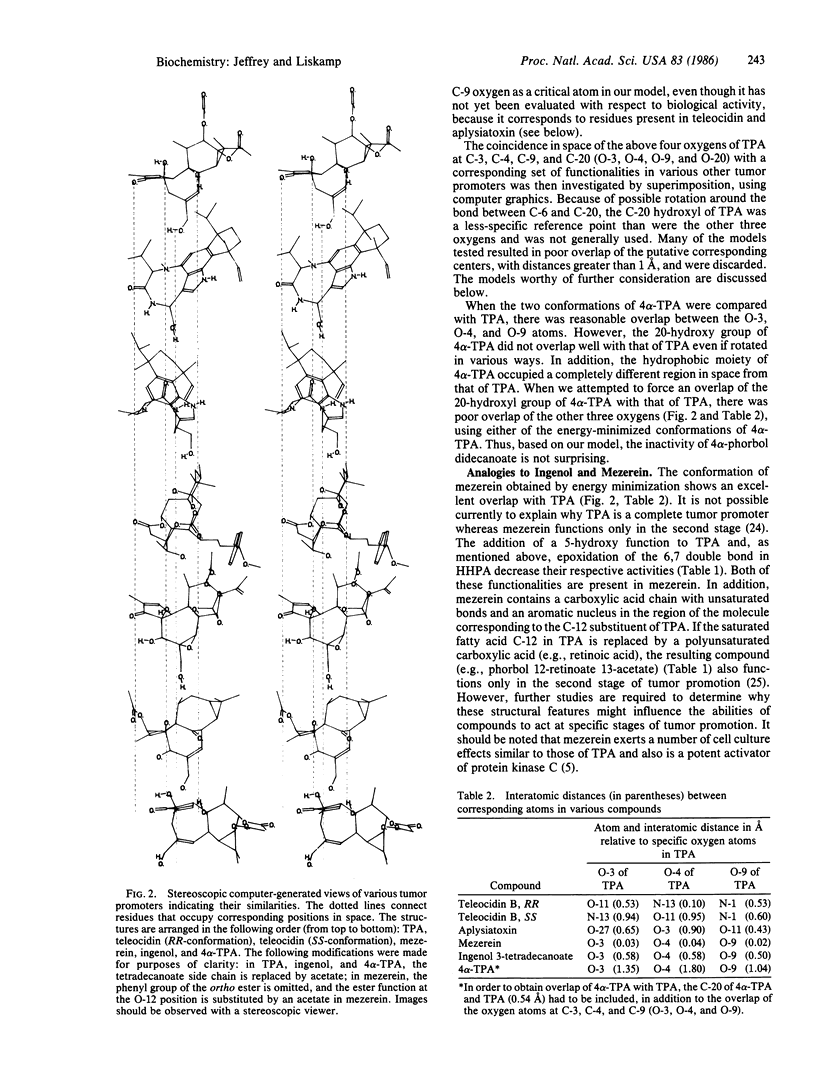
In the two-stage model of skin carcinogenesis, it is believed that initiators bind to DNA and that tumor promoters such as phorbol 12-tetradecanoate 13-acetate (TPA) bind noncovalently to membrane-associated high-affinity receptors, probably protein kinase C. Two other types of potent tumor-promoting substances, aplysiatoxin and teleocidin, appear to act also by binding to and activating protein kinase C, even though their chemical structures are quite different. Therefore, we have undertaken computer modeling of the special relationship of various functional groups in these three chemical classes of tumor promoters in an attempt to explain how these diverse structures bind to the same receptor molecule. We propose a stereochemical model in which the oxygens in TPA at C-3, C-4, C-9, and C-20 (O-3, O-4, O-9, and O-20) correspond to the O-11, N-13, N-1, and O-24 positions in teleocidin and the O-27, O-3, O-11, and O-30 oxygens in aplysiatoxin, respectively. In this model all distances with respect to overlap of the corresponding atoms are less than 1 A. In addition, all three types of molecules have their hydrophobic moieties oriented in a similar position. This model is further discussed with respect to other compounds showing various degrees of activity as tumor promoters, including mezerein, ingenol, and 4 alpha-TPA. The model explains how chemically diverse structures can have similar biological activity as tumor promoters and provides a basis for designing both agonists and antagonists of tumor promoters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcoleo J. P., Weinstein I. B. Activation of protein kinase C by tumor promoting phorbol esters, teleocidin and aplysiatoxin in the absence of added calcium. Carcinogenesis. 1985 Feb;6(2):213–217. doi: 10.1093/carcin/6.2.213. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Cardellina J. H., 2nd, Marner F. J., Moore R. E. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979 Apr 13;204(4389):193–195. doi: 10.1126/science.107586. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Delclos K. B., Yeh E., Blumberg P. M. Specific labeling of mouse brain membrane phospholipids with [20-3H]phorbol 12-p-azidobenzoate 13-benzoate, a photolabile phorbol ester. Proc Natl Acad Sci U S A. 1983 May;80(10):3054–3058. doi: 10.1073/pnas.80.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Matsukura N., Sugimura T., Takayama S. Teleocidin from Streptomyces is a potent promoter of mouse skin carcinogenesis. Carcinogenesis. 1982;3(8):895–898. doi: 10.1093/carcin/3.8.895. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Nakayasu M., Tahira T., Endo Y., Shudo K., Sugimura T. Structure-activity studies on synthetic analogues (indolactams) of the tumor promoter teleocidin. Gan. 1984 Oct;75(10):866–870. [PubMed] [Google Scholar]
- Fujiki H., Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y., Sugimura T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: teleocidin and debromoaplysiatoxin. Biochem Biophys Res Commun. 1984 Apr 30;120(2):339–343. doi: 10.1016/0006-291x(84)91259-2. [DOI] [PubMed] [Google Scholar]
- Fürstenberger G., Berry D. L., Sorg B., Marks F. Skin tumor promotion by phorbol esters is a two-stage process. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7722–7726. doi: 10.1073/pnas.78.12.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M., Horn F., Kittstein W., Fürstenberger G., Marks F. Soluble phorbol ester binding sites and phospholipid- and calcium-dependent protein kinase activity in cytosol of chick oviduct. FEBS Lett. 1983 Oct 3;162(1):147–150. doi: 10.1016/0014-5793(83)81067-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Fujiki H., Suganuma M., Hakii H., Nakayasu M., Hitotsuyanagi Y., Aimi N., Sakai S., Endo Y., Shudo K. Studies on olivoretins indicate a requirement for a free hydroxyl group for teleocidin B activity. Gan. 1984 Oct;75(10):837–840. [PubMed] [Google Scholar]
- Horowitz A. D., Fujiki H., Weinstein I. B., Jeffrey A., Okin E., Moore R. E., Sugimura T. Comparative effects of aplysiatoxin, debromoaplysiatoxin, and teleocidin on receptor binding and phospholipid metabolism. Cancer Res. 1983 Apr;43(4):1529–1535. [PubMed] [Google Scholar]
- Horowitz A. D., Greenebaum E., Weinstein I. B. Identification of receptors for phorbol ester tumor promoters in intact mammalian cells and of an inhibitor of receptor binding in biologic fluids. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2315–2319. doi: 10.1073/pnas.78.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Adolf W., Marston A., Roeser H., Sorg B., Fujiki H., Sugimura T., Moore R. E., Hecker E. Inhibition of specific binding of [3H]phorbol-12,13-dipropionate to an epidermal fraction by certain irritants and irritant promoters of mouse skin. Carcinogenesis. 1983;4(1):77–81. doi: 10.1093/carcin/4.1.77. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Heck K., Sorg B., Hecker E. Phospholipids involved in specific binding of 12-O-(5-azido-2-nitrobenzoyl)phorbol-13-acetate to epidermal microsomes, a photolabeling study. Cancer Lett. 1985 Feb;26(1):97–111. doi: 10.1016/0304-3835(85)90178-8. [DOI] [PubMed] [Google Scholar]
- Shimomura K., Mullinix M. G., Kakunaga T., Fujiki H., Sugimura T. Bromine residue at hydrophilic region influences biological activity of aplysiatoxin, a tumor promoter. Science. 1983 Dec 16;222(4629):1242–1244. doi: 10.1126/science.6316505. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Weeks C. E., Nelson K., Mamrack M., Klein-Szanto A. J. Specificity and mechanism(s) of promoter inhibitors in multistage promotion. Carcinog Compr Surv. 1982;7:19–34. [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Tahira T., Cheuk C., Moore R. E., Sugimura T. Estimation of tumor promoting activity and structure-function relationships of aplysiatoxins. Carcinogenesis. 1984 Mar;5(3):315–318. doi: 10.1093/carcin/5.3.315. [DOI] [PubMed] [Google Scholar]


