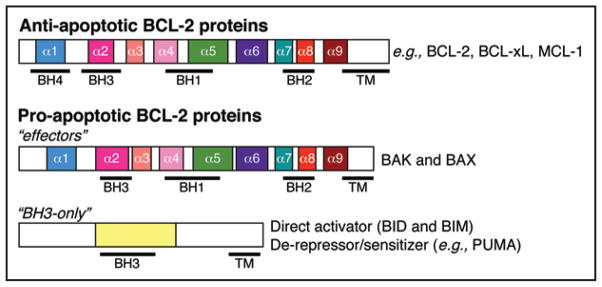
Figure 1.
The BCL-2 family of proteins is divided into three functional groups based on their composition of BCL-2 homology (BH) domains. The anti-apoptotic members (e.g., BCL-2, BCL-xL and MCL-1) contain four BCL-2 homology domains (designated BH1-4). The pro-apoptotic effectors (BAK and BAX) contain BH1-3 domains. The BH3-only proteins are structurally diverse and contain only one conserved region, the BH3 (leucine-x-x-x-x-aspartic acid, x = any amino acid). The BH3-only proteins are subdivided into direct activators (BID and BIM) and de-repressors/sensitizers (e.g., PUMA). The alpha helices of each protein are designated, and the regions contained within each BH domain are illustrated by bold lines under each protein. The hydrophobic carboxyl terminal transmembrane domain (TM) of each protein is based on in silico predictions and/or structural data and is not necessarily present in each member.