Elite suppressors (ES) represent a unique population of HIV infected persons who control HIV replication below detectable levels without antiretroviral therapy. This group constitutes less than 1% of the HIV-infected population 1, and there have been few comparisons with HIV-uninfected persons and other HIV-infected groups 1–4. Previous research has shown that, regardless of host-mediated viral containment, some ES demonstrated continuous CD4+ T cell loss and increased T cell activation 1. One of the mechanisms that may contribute to T cell loss is activation-induced cell death (AICD) of CD4+ T cells 5. AICD is one of two apoptotic pathways that trigger the activation of caspase-3 6. Caspase-3, a cysteine protease, is a pro-apoptotic marker involved in the signaling cascade of cellular apoptosis, and is activated downstream upon its cleavage by other caspases 7–9. In previous studies involving antigen-stimulation of CD4+ T cells, caspase-3 expression correlated directly with HIV-1 viral load and inversely with CD4 count 6. Increased AICD in untreated HIV-infected individuals has been reported and is the likely mechanism of CD4+ T cell loss in HIV disease 5. This study was undertaken to understand if naturally controlled ES were immunologically different from subjects that contained the virus through effective HAART.
This study was nested within the Women’s Interagency HIV Study (WIHS), an ongoing multi-site prospective study of HIV among US women 10. Semiannual visits include interview, clinical exam, and collection of biologic specimens. Multi-parameter flow cytometry was utilized to measure intracellular expression of activated caspase-3 and surface expression of T cell activation markers (HLA DR+CD38+) in CD4+ and CD8+ T cells from 3 groups of participants: (i) ES (n=10), women with at least 1.5 years of HIV RNA ≤80 copies/ml in the absence of antiretroviral therapy 1; (ii) successfully treated HIV+ (n=9), women on antiretroviral therapy with suppressed HIV RNA (≤80 copies/mL) and CD4 counts≥350 cells/mL were chosen as a comparator group to study the impact of highly active antiretroviral therapy (HAART) versus natural control in ES; and (iii) HIV-uninfected controls (n=6). Of the 25 study participants, 11 (44%) were African-American and 7 (28%) were Caucasian; the median age was 39 years (interquartile range: 31–45). Study groups did not differ significantly by race (p>0.05). HIV-uninfected controls (median age 24 years) were younger, and ES had a significantly lower median CD4+ cell count than successfully treated participants (521 vs. 714 cells/ml, p=0.03). The ES and successfully treated controls did not differ by age (median 41.5 vs. 44 years, p>0.05).
The cryo-preserved peripheral blood mononuclear cells (PBMCs) were thawed, rested overnight at 37°C in an incubator supplied with 5% CO2. The next day the cells were stained by first adding LIVE/DEAD® Fixable Aqua stain (Molecular Probes, Inc., Eugene, OR) to identify non-viable cells, followed by monoclonal antibodies: Pacific Blue-conjugated anti-CD3 (BD Pharmingen™, San Jose, CA), PE-Texas Red-conjugated anti-CD4-PE Texas Red (Invitrogen, Carlsbad, CA), and APC-H7-conjugated anti-CD8 (BD Biosciences, San Jose, CA) for T cell subset analysis, and PE-Cy7-conjugated anti-HLA-DR (BD Biosciences) and PerCP-Cy™5.5-conjugated anti-CD38 (BD Pharmingen™) for T cell activation. The cells were permeabilized prior to intracellular staining with FITC-conjugated anti-active Caspase-3 (BD Pharmingen™) for pro-apoptotic expression analysis. The cells were fixed with 2% formaldehyde. Flow cytometry acquisition was performed on a BD LSRII (BD Biosciences) instrument and analyzed by FlowJo (Tree Star, Inc., Ashland, OR). Nonparametric analysis of variance (ANOVA) with Dunn’s multiple comparisons was conducted as the sample size was small and data were non-normally distributed.
Our results demonstrated significantly higher caspase-3 expression in ES versus the HIV-uninfected controls in both CD4+ (p<0.05) and CD8+ (p<0.01) T cells (Fig. 1a). Similarly, the successfully treated HIV+ women had significantly elevated levels of caspase-3 in both CD4+ (p<0.05) and CD8+ (p<0.01) T cell subsets compared to the HIV-uninfected controls. No significant differences were observed between the two HIV-infected groups.
Figure 1. Elevated caspase-3 and T cell activation in Elite suppressors (ES).
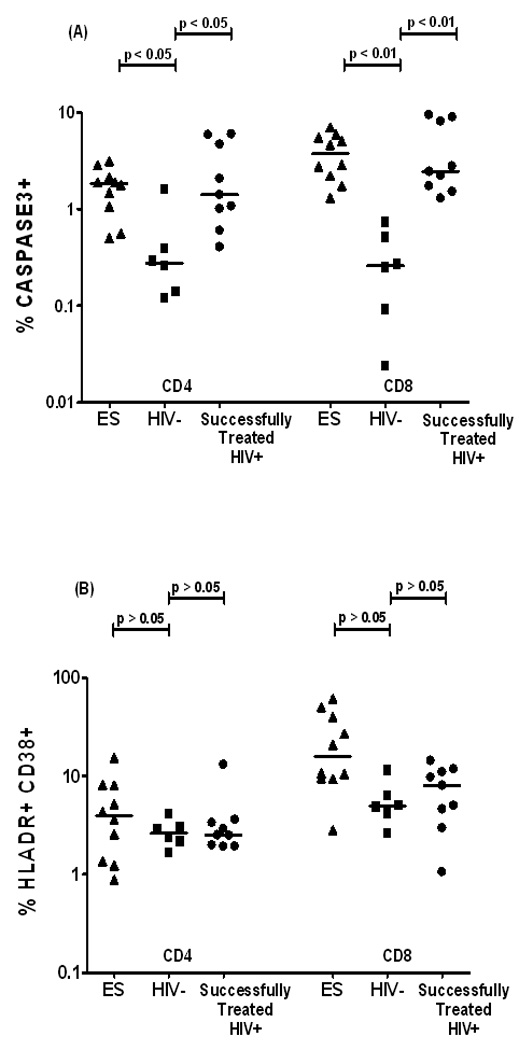
Scatter plots exhibit the % expression of spontaneous activated Caspase-3 (A) and HLA DR+CD38+ (B) in CD4+ and CD8+ T cells from PBMCs of HIV-uninfected controls (HIV, n=6), elite suppressors with VL≤80 (ES, n=10) and HIV-infected individuals on optimally suppressive antiretroviral therapy with VL≤80 CD4 ≥350 cells/mL (successfully treated HIV+, n=9).
Both CD4+ and CD8+ T cell activation levels were higher among ES as compared to the uninfected controls. Successfully treated HIV+ women had higher CD8 T cell activation compared to uninfected controls (Fig. 1b). However, this difference did not attain statistical significance (p>0.05).
Overall, observations from this study indicate that both ES and successfully treated HIV+ subjects exhibited significantly elevated spontaneous T cell caspase-3 expression compared to HIV-uninfected controls. This increased level of caspase-3 expression may be due to residual HIV replication among both ES and HAART treated subjects. As previously demonstrated using a single copy assay both these groups had equivalent low levels of HIV.11 In conclusion the increased level of caspase- 3 expression in CD4 T cells of ES coupled with decreased CD4 levels compared to HAART treated subjects suggests that ongoing low level of viral replication may ultimately contribute to immune decline via AICD. Immune restoration in these natural controllers may require antiretroviral therapy or other immune modulatory treatment.
Acknowledgements
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Funding:
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health
References
- 1.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009 Jan;83(1):329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008 Sep;82(17):8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emu B, Sinclair E, Favre D, et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005 Nov;79(22):14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. Aids. 2009 Jun 1;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dockrell DH, Badley AD, Algeciras-Schimnich A, et al. Activation-induced CD4+ T cell death in HIV-positive individuals correlates with Fas susceptibility, CD4+ T cell count, and HIV plasma viral copy number. AIDS Res Hum Retroviruses. 1999 Nov 20;15(17):1509–1518. doi: 10.1089/088922299309793. [DOI] [PubMed] [Google Scholar]
- 6.Yue FY, Kovacs CM, Dimayuga RC, et al. Preferential apoptosis of HIV-1-specific CD4+ T cells. J Immunol. 2005 Feb 15;174(4):2196–2204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 7.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999 Feb;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 8.Stennicke HR, Jurgensmeier JM, Shin H, et al. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998 Oct 16;273(42):27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 9.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999 Jul 16;274(29):20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 10.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998 Mar;9(2):117–125. [PubMed] [Google Scholar]
- 11.Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008 Jul 1;47(1):102–104. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]


