Figure 1.
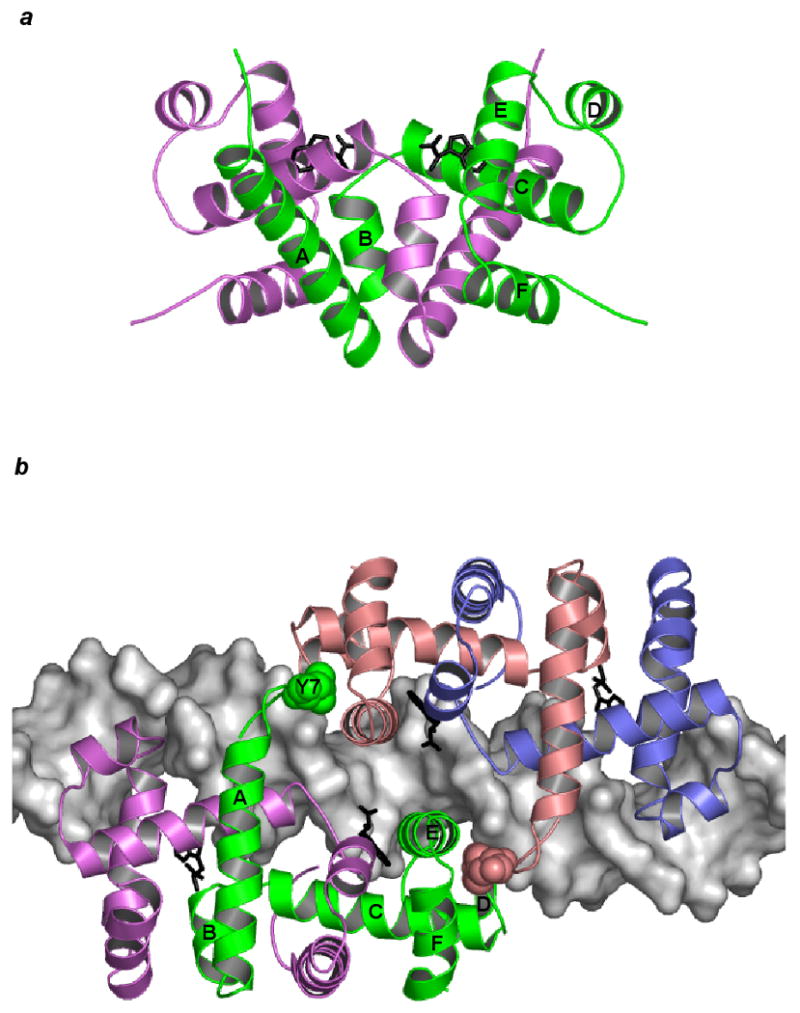
Dimeric trpR. (a) The trpR dimer is shown in schematic ribbon view. Two equivalent subunits are represented in green and purple (orthorhombic crystal form, PDB entry 2WRP (Lawson et al., 1988)). Two L-tryptophan corepressors (black) fill hydrophobic pockets within the extensive interface between the intertwined subunits. (b) Two trpR dimers are shown bound to DNA at tandem sites spaced 8 base-pairs apart (left dimer: green and purple subunits, right dimer: pink and blue subunits, tandem trpR-DNA complex, PDB entry 1TRR (Lawson and Carey, 1993)). For the subunits participating in tandem association between the two dimers (left dimer: green, right dimer: pink), flexible N-terminal arm residue Tyr 7 is displayed in space-fill and labeled on the green subunit. In both (a) and (b), helices A-F of the green subunit are labelled.
