Figure 2.
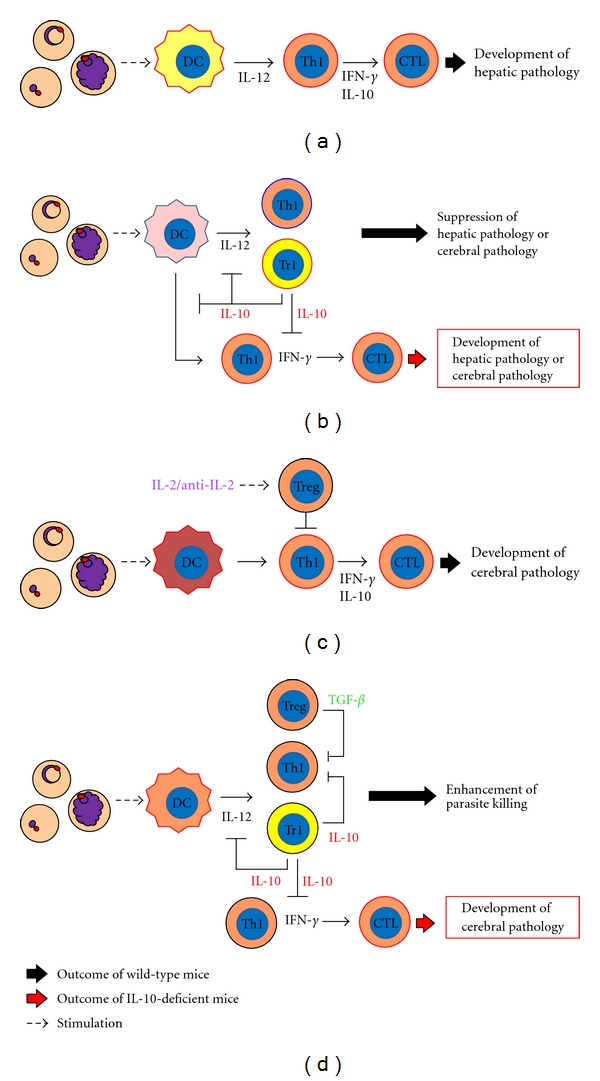
Role of anti-inflammatory responses during murine malaria infection. (a) Immune responses during lethal P. berghei NK65 infection. (b) Possible mechanism by which development of disease severity such as hepatic or cerebral pathology is suppressed by coinfection with nonlethal murine malaria parasites. (c) Immune responses during lethal P. berghei ANKA infection. (d) Immune responses during P. chabaudi AS infection. (a) and (c) A type of DC activated by lethal P. berghei NK65 or P. berghei ANKA might induce pathological Th1 and CTL. The pathological Th1 and CTL are involved in excessive inflammation and the development of severe pathology, such as hepatic pathology (P. berghei NK65) or experimental cerebral malaria (ECM) (P. berghei ANKA). (b) A type of DC activated by nonlethal malaria parasites before activation by lethal parasites might expand Tr1, but not pathological Th1, and might subsequently suppress severe disease. IL-10, which might be produced by Tr1, inhibits proinflammatory cytokine production and expansion of pathological Th1 during coinfection. Therefore, coinfected IL-10 deficient mice develop severe pathology, such as hepatic pathology or ECM. (d) In P. chabaudi AS infection, IL-10 from Tr1 and Treg are associated with suppression of proinflammatory cytokine production and expansion of pathological Th1. A deficiency of IL-10 or TGF-β contributes to development of ECM.
