Abstract
Chromosomal translocations and activation of the FGF receptor FGFR1 are a feature of stem cell leukemia-lymphoma syndrome (SCLL), an aggressive malignancy characterized by rapid transformation to acute myeloid leukemia and lymphoblastic lymphoma. It has been suggested that FGFR1 proteins lose their ability to recruit Src kinase, an important mediator of FGFR1 signaling, as a result of the translocations that delete the extended FGF receptor substrate-2 (FRS2) interacting domain that Src binds. In this study, we report evidence that refutes this hypothesis and reinforces the notion that Src is a critical mediator of signaling from the FGFR1 chimeric fusion genes generated by translocation in SCLL. Src was constitutively active in BaF3 cells expressing exogenous FGFR1 chimeric kinases cultured in vitro as well as in T-cell or B-cell lymphomas they induced in vivo. Residual components of the FRS2 binding site retained in chimeric kinases that were generated by translocation were sufficient to interact with FRS2 and activate Src. The Src kinase inhibitor dasatinib killed transformed BaF3 cells and other established murine leukemia cell lines expressing chimeric FGFR1 kinases, significantly extending the survival of mice with SCLL syndrome. Our results indicated that Src kinase is pathogenically activated in lymphomagenesis induced by FGFR1 fusion genes, implying that Src kinase inhibitors may offer a useful option to treat of FGFR1-associated myeloproliferative/lymphoma disorders.
Introduction
Human stem cell leukemia-lymphoma syndrome (SCLL), also known as 8p11 myeloproliferative syndrome (EMS), is a rare atypical myeloproliferative disorder (MPD) (1). SCLL expresses a clinical phenotype with features of both lymphoma and sometimes eosinophilic myeloproliferative disorders. SCLL is characterized by a reciprocal chromosome translocation (2) resulting in a chimeric protein which activates the kinase domain of the fibroblast growth factor receptor-1 (FGFR1) (3-5). To date, at least 11 different gene partners have been shown to fuse to FGFR1, including ZMYM2 (formerly ZNF198) on 13q12, BCR on 22q11 and CEP110 on 9q33 (6) and the recently described CUX1-FGFR1 involving 7q22 (7). The t(8;13) (p11;q12) rearrangement is the most commonly observed translocation in SCLL, in which the zinc finger domain of ZMYM2 is fused to the intracellular kinase domain of FGFR1. The clinical course of SCLL is aggressive, with rapid transformation to acute myeloid leukemia (AML) and lymphoblastic lymphoma of common T-cell origin (8-10). Treatment with conventional chemotherapy is often not effective (9), and allogeneic bone marrow transplantation offers the only potentially curative therapeutic option (11).
FGFR1 belongs to a large group of protein tyrosine kinases that play crucial roles in controlling cell growth, differentiation and survival, among other functions (12). Like all receptor tyrosine kinases (RTKs), the FGF receptors comprise an extracellular ligand binding domain, a single transmembrane region and a cytoplasmic domain composed of a protein tyrosine kinase core. Upon ligand binding, FGFR1 normally undergoes rapid auto-phosphorylation of several tyrosine residues. Phospho-activation of FGFR1 results in tyrosine phosphorylation of downstream targets such as phospholipase C-gamma (PLCg) and the FGF receptor substrate (FRS2) docking protein (13). Activated FRS2 can, in turn, trigger the Ras/MAPK kinase signaling cascade (13-14). It has been shown that Src is also recruited by activated FGFR1 through FRS2 (15), which plays an important role in FGFR1 mediated endothelial cell differentiation (16). Here we show that activation of Src was frequently seen in all FGFR1 chimeric kinase-transformed BaF3 cells, as well as lymphomas induced in vivo by ZMYM2-FGFR1, BCR-FGFR1, and CEP110-FGFR1. Inhibition of Src by Dasatinib can significantly reduce growth of FGFR1 fusion-associated leukemia cells in vitro and delays their tumorigenesis in vivo. Our data indicate that pharmacologic inhibition of FGFR1 fusion kinases with Dasatinib may be effective in treatment of myeloproliferative disorders associated with chimeric FGFR1 kinases and perhaps for other human disorders associated with dysregulated FGFR1 activity.
Materials and methods
Cell culture and proliferation assays
All cell lines were cultured in RPMI (Invitrogen) with 10% FBS (Hyclone), at 37°C in 10% CO2. For drug treatments, 40,000 cells/well were seeded in 96-well plates and incubated overnight, then treated with the either DMSO (control) or the drugs indicated in the results section at concentrations defined by the experiments. Cell viability was determined using Cell Titer-Glo luminescence cell viability kits (Promega) and a SpectraMax® M5e (Molecular Probe) luminescence plate reader. CellTrace Violet (Invitrogen) or PKH26 (Sigma) proliferation kits were used to track cell division. In these approaches cells are initially labeled with specific fluorchromes. As the cells divide the fluorochrome is distributed to the daughter cells and so the intensity of fluorescence in the population declines at a rate proportional to the rate of cell proliferation.
Transduction and infection
The dominant negative mouse Src K295R/Y527F construct (Addgene) in pCMV5 was subcloned into the YFP pMIYII vector (a kind gift from Dr. Dario Vignali, St. Jude Children’s Research Hospital, Memphis, TN) and designed as pMIY-DNSrc. The presence of mutations was confirmed by sequencing. The procedure for transduction and infection was as described previously (17). Supernatants containing retroviral particles of the empty pMIYII vector and pMIY-DNSrc were generated using Phoenix package cells as described previously (17). Cells were infected with the respective supernatant 4 times. The infected cell pools were used for apoptosis assays one week after infection.
Cell apoptosis assay, cell cycle and phospho-specific flow cytometry
For analysis of apoptosis, cells were stained with Annexin V and 7-AAD (BD Biosciences) following the manufacturer’s protocol. Appearance of Annexin V and 7-AAD in flow cytometric analysis indicated onset of apoptosis. Cell cycle was analyzed by Flow cytometry using standard procedures following propidium iodide staining. For intracellular staining of phospho-Src following drug treatment, cells were fixed and permeabilized before reacting with a phospho-Src antibody (BD Biosciences) according to standard procedures. Cells were analyzed using a LSRII flow cytometer. All flow cytometry data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Immunoprecipitation and western blot analyses
Proteins were isolated using standard Trizol procedures. For immunoprecipitations, 1500 μg of cell lysates were incubated with the FRS2 antibody (Sigma) at 4°C over night, followed by addition of protein A/G-agarose beads (Pierce, Rockford, IL). Immunoprecipitates, or whole-cell lysates (50 μg), were separated using SDS-PAGE and immunoblotted with the following specific antibodies: anti-FGFR1 and GAPDH (Santa Cruz), anti-Src and anti-pSrc (Cell signaling) and beta-actin (Sigma) using standard protocols.
Anchorage independent growth
Cells were suspended in DMEM containing 10% FCS and 0.3% agar and then seeded into 6-well plates pre-coated with 0.6-1.0% agar in DMEM and 10% FBS. Twenty thousand cells were seeded in triplicate wells. Medium was changed twice a week. Colonies were counted at 5× magnification 3 weeks post-seeding.
Animals and treatment schedule
Female Balb/c mice (Taconic, 6-8 weeks old) were injected with ZNF112 or CEP2A cells at 2 × 106 per mouse through the tail vein. One week after injection, mice were randomized to treatment with vehicle control or Dasatinib (ChemieTek, Indianapolis, Inc.). ZNF112 transplanted mice were divided into 3 groups: Control (n=10), Dasatinib 20 mg/kg (n=10) and dasatinib 40 mg/kg (n = 10). CEP2A mice were divided into 2 groups: Control (n=13) or Dasatinib 40 mg/kg (n=10). Dasatinib was dissolved in 80 mM citrate buffer (pH 3.1) (18), and given orally using a gavage needle twice per day. The control group of mice was given an equal volume of the citrate buffer by gavage. All treatments were performed 6 days per week for 4 weeks. All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia.
Human CD34+ cord blood cell culture and infection
Human cord blood cells were obtained from the Cord Blood Bank of the Medical College of Georgia under approved IRB protocols. CD34+ cells were isolated using the EasySep Cord Blood CD34 Positive Selection Kit (StemCell Technologies) following the manufacturer’s protocol, and expanded in StemSpan SFEM medium (StemCell) supplemented with recombinant human cytokines: LDL 10ug/ml, Flt-3 100ng/ml, SCF 100ng/ml, TPO 50ng/ml, Il-3 20ng/ml and IL6 20ng/ml (R & D Systems). After 24h pre-stimulation, CD34+ cells were infected with either BCR-FGFR1 or the control MIEG3 vector as described previously (17).
Results
Transformation BaF3 cells by ZMYM2-FGFR1, BCR-FGFR1 or CEP110-FGFR1 is associated with Src activation
It was previously suggested that chimeric FGFR1 proteins had lost their ability to recruit Src kinase due to loss of the FRS2 binding site in the FGFR1 fusion proteins (19). However, it has also been demonstrated that 8/9 members of the Src family kinases were highly phosphorylated in the human KG-1 myeloid cell line, which express the FGFR1OP2-FGFR1 fusion kinase (20). Activation of Src kinase plays a crucial role in the normal process of FGFR1 signaling dynamics (14-15). This observation suggests that either the chimeric FGFR1 proteins can still recruit FSR2, or that Src family kinases are activated indirectly in the presence of the FGFR1 fusion kinases. Based on the recently updated sequence of the FGFR1 gene (NCBI: NM_023110.2), the breakpoint in all FGFR1-associated translocations occurs within intron 12 (previously described as intron 8). As a result, all chimeric FGFR1 proteins contain amino acids 429-822 (based on FGFR1 transcript variant 1) which retains part of the FRS2 binding site (Fig. 1A) (21-22). The extended FRS2 binding site involves amino acids 420-433 (21-22), although a 5′ truncated FRS2 binding site retained is in the chimeric FGFR1 proteins (Fig. 1A), which raised the possibility that the FGFR1 fusion proteins may, in fact, be able to recruit the FRS2 adaptor protein. If this is the case for the FGFR1-fusion kinases, then constitutive activation of Src kinases may play an important role in SCLL-associated leukemogenesis. To investigate this possibility, we first determined whether Src activation could be detected in cells expressing exogenous FGFR1 fusion kinases.
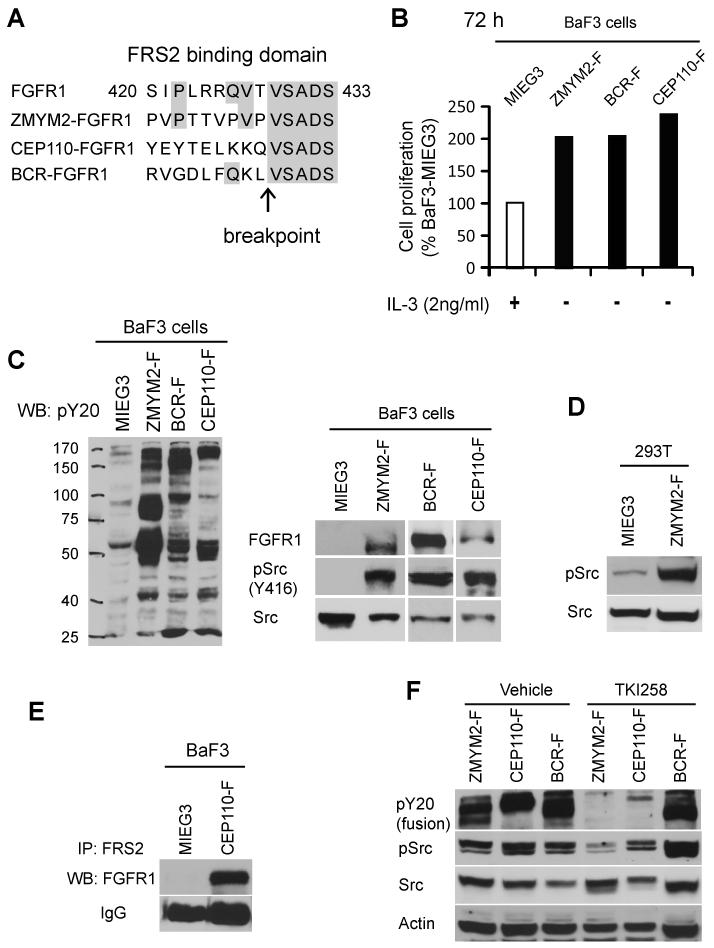
Figure 1. Activation of Src is associated with FGFR1 chimeric kinese expression.
A) Schematic outline of the FGFR1 break point junction in the chimeric FGFR1 fusion kinases shows conserved amino acids within the FRS2 consensus binding domain (amino acids 420-433) (21-22). (B) Cell proliferation rates in BaF3 cells transformed by ZMYM2-FGFR1 (ZMYM2-F), BCR-FGFR1 (BCR-F) or CEP110-FGFR1 (CEP110-F) show both IL-3 independence and an increased growth rate relative to cells carrying the empty vector (MIEGS). (C) Transduction of BaF3 cells (left panel) with the chimeric FGFR1 kinases identifies constitutive tyrosine phosphorylation in total cellular proteins using the anti-PY20 antibody compared with cells carrying the empty vector. These cells also show phospho-activation of Src kinase (right panel). (D) Ectopic expression of ZMYM2-FGFR1 in adherent 293T cells also leads to increased levels of phospho-Src. (E) Immunoprecipitation using anti-FRS2 antibodies co-precipitates the CEP110-FGFR1 fusion protein in CEP110-FGFR1 (CEP110-F) transformed BaF3 cells, but not in cells transduced with the empty vector. (F) When BaF3 cells expressing either ZMYM2-FGFR1 or CEP110-FGFR1 are treated with the TKI258 (400 nM) FGFR1 inhibitor, activation of both the FGFR1 fusion kinases and Src is inhibited. In contrast, TKI258 does not affect activation of either BCR-FGFR1 or Src in BCR-FGFR1 transformed cells.
BaF3 murine, pro-B lymphoma cells normally require IL-3 supplements for growth, and oncogenic transformation results in IL3 independent growth. Following forced expression of either ZMYM2-FGFR1, BCR-FGFR1 or CEP110-FGFR1 (from retroviral constructs co-expressing GFP), BaF3 cells became IL-3 independent in all cases (Fig. 1B), compared with BaF3 control cells transfected with the empty vector (MIEG3) which died within 24h in the absence of IL-3 (data not shown). These observations indicated that these three FGFR1 fusion genes can successfully transform BaF3 cells. We then compared the growth rate of transformed BaF3 cells in the absence of IL-3 with that of MIEG3-BaF3 (GFP sorted) cells in the presence of IL-3. BaF3 cells transformed with all three fusion kinases proliferated significantly faster than MIEG3-BaF3 cells (Fig. 1B). Western blot analysis confirmed high expression levels of ZMYM2-FGFR1, BCR-FGFR1, and CEP110-FGFR1 in the transformed BaF3 cells (Fig. 1C). As expected, constitutive activation of the chimeric kinases was associated with significantly higher global tyrosine phosphorylation levels compared with MIEG3-BaF3 cells (Fig. 1C). High levels of activated Src were also observed in these cells using western blot analysis with an antibody that recognizes Y416-activated Src (Fig. 1C). In addition, ectopic expression of the ZMYM2-FGFR1 chimeric kinase in adherent 293T cells, which have been used extensively to study chimeric FGFR1 signaling (23-24), also induced Src activation (Fig. 1D). Next, we used representative BaF3-CEP110-FGFR1 and BaF3-MIEG3 cells to determine whether FRS2 interacts with the FGFR1 fusion protein. Following immunoprecipitation with an anti-FRS2 antibody, western blot analysis clearly shows that FRS2 can co-IP with the CEP110-FGFR1 fusion protein (Fig. 1E). Finally, when transformed BaF3 cells were treated with the TKI258 FGFR1 inhibitor (25), the levels of phospho-FGFR1 were dramatically reduced, with a concomitant decrease of Src phosphorylation (Fig. 1F). Together, these data demonstrate that expression of all 3 fusion proteins is associated with Src activation in stably transformed BaF3 cells.
Inhibition of Src activation in FGFR1 fusion transduced BaF3 cells inhibits cell proliferation
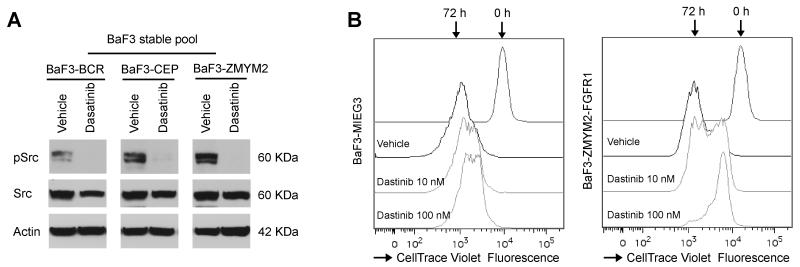
To determine whether inhibiting Src activation affects cell growth in FGFR1 chimeric kinase-expressing cells, we treated BaF3 cells transformed with the three chimeric kinases with Dasatinib for 24h at their GI50 doses. Western blot analysis shows that Dasatinib can demonstrably reduce phospho-Src levels (Fig. 2A). Using CellTrace Violet Fluorescence to monitor cell proliferation, we then demonstrated that growth of the IL3 independent cells was reduced by 50% following treatment with 10 nM Dasatinib for 72 h (Fig. 2B). Growth rate was even slower using 100 nM Dasatinib (Fig. 2B). The same treatments did not significantly affect growth of BaF3 cells carrying the empty vector (Fig. 2B).
Figure 2. Downregulation of activated Src correlates with growth inhibition.
(A) Dasatinib treatment (100 nM) leads to suppression of Src kinase autophosphorylation in cells expressing ZMYM2-FGFR1, CEP110-FGFR1 and BCR-FGFR1. (B) Flow cytometric tracking of cell division with CellTrace™ Violet shows that 72 hours exposure to Dasatinib reduces cell proliferation in cells expressing ZMYM2-FGFR1 but not cells carrying the empty MIEG3 vector or treated with DMSO (vehicle).
Src activation occurs in primary lymphomas induced by FGFR1 fusion genes in vivo
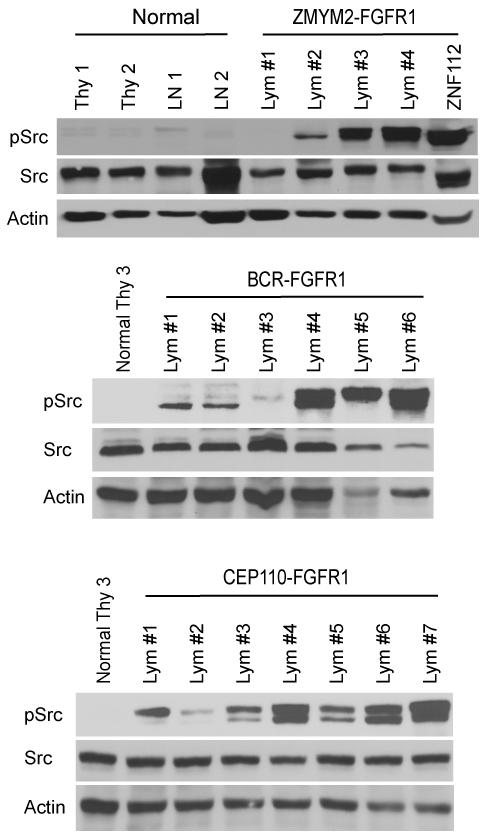
We previously described a murine model for ZMYM2-FGFR1 SCLL using a bone marrow transduction and transplantation approach (17). These mice developed T-lymphoblastic lymphoma with a double positive, CD4+/CD8+, immunophenotype. We have since also developed murine models for BCR-FGFR1 and CEP110-FGFR1 SCLL. BCR-FGFR1 induces leukemias with a CD19+/B220+/- immunophenotype that is consistent with pro-B lymphoma (Tidwell et al. manuscript submitted). In the CEP110-FGFR1 SCLL model, mice developed both T-cell and B-cell lymphomas (Ren et al. manuscript in preparation). Mice in all 3 FGFR1-associated SCLL models concomitantly develop myeloid lineage disease. Here we focused on the T- and B-lymphomas from these mouse models. Western blot analysis using an anti-phospho-Src (Y416) antibody clearly shows high levels of Src activation in the majority of lymphomas from the 3 in vivo FGFR1-associated SCLL models (Fig. 3), suggesting that constitutive activation of Src could be involved in the etiology of the lymphomagenesis induced by the chimeric FGFR1 genes.
Figure 3. Src is activated in primary lymphomas.
Western blot analyses of phospho-activated and total Src levels in normal thymocytes (Thy) and lymph nodes (LN) from normal Balb/c mice compared with cells derived from lymph nodes (Lym) from mice with leukemia/lymphoma from the three different fusion kinase models. In these experiments Src activation is seen in the majority of primary tumors from the three different models.
Src activation in stable cell lines from leukemia mice and inhibition of Src activation induce cell death
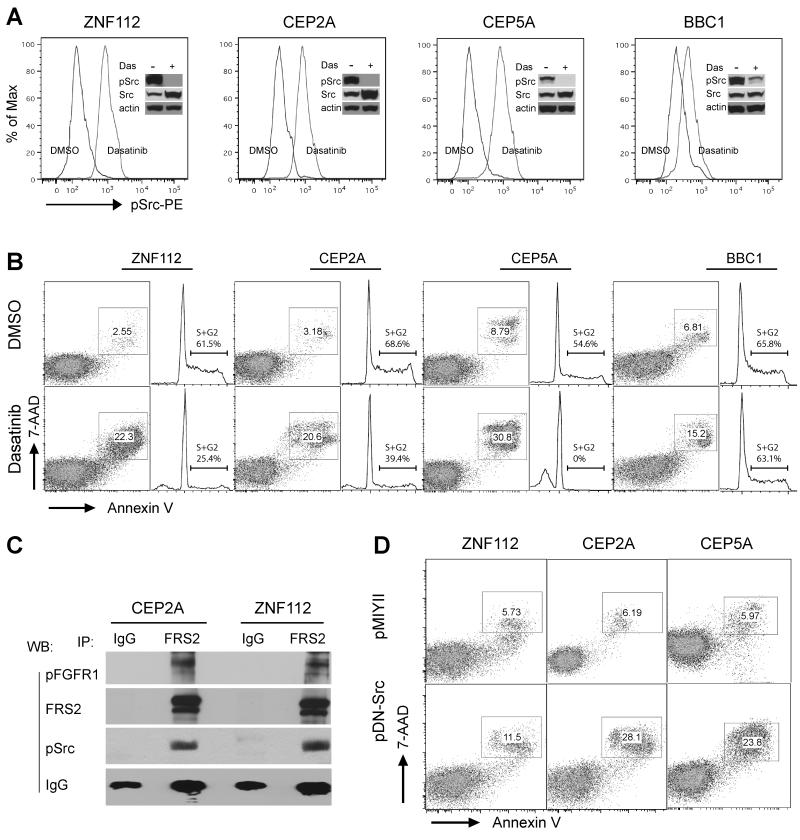
Recently, we generated several cell lines from the three different SCLL mouse models. ZNF112 carries the ZMYM2-FGFR1, BBC1 carries the BCR-FGFR1 rearrangement and CEP2A and CEP5A carry CEP110-FGFR1. ZNF112, CEP2A and CEP5A cells are derived from T-cell lymphomas. BBC1 cells have pro-B cell immunophenotypes. Both western blot and phospho-flow analyses demonstrated high levels of activated Src in these cell lines (Fig. 4A). When ZNF112, CEP2A, CEP5A and BBC1 cells were treated with Dasatinib in the 300-1000 nM range for 24h, Phospho-flow and western blot analyses demonstrated an almost complete loss of phospho-Src (Fig. 4A). Consequently, these cells undergo apoptosis and cell death (Fig. 4B) with a comcomitant reduction in the passage of cells through the cell cycle, demonstrating that, for these cells, activated Src is a critical survival factor. Similar to the chimeric FGFR1 transformed BaF3 cells (Fig. 1E), immunoprecipitation with the anti-FRS2 antibody clearly shows FRS2 can co-IP with both the phosphorylated FGFR1 fusion protein and Src kinase (Fig. 4C) in ZNF112 and CEP2A cells, while immunoprecipitation with the rabbit IgG isotype did not. To further exclude the non-specific effects of Dasatinib, we infected ZNF112, CEP2A and CEP5A cells with retrovirus constructs carrying either a dominant negative Src (pDNSrc) (26) or control vector (pMIYII). One week post infection, flow cytometry analysis demonstrated 2-4 times greater cell death (double positive of Annexin V and 7-AAD) in the pDNSrc cells compared with the pMIYII cells (Fig. 4D). Taken together, these observations demonstrate that the chimeric FGFR1 may drive tumorigenesis through activation of Src kinase.
Figure 4. Inhibition of Src activation results in apoptotic cell death.
(A) Flow cytometric and western blot analysis of activated Src shows reduced phospho-Src levels in BaF3 cells stably expressing the three chimeric FGFR1 kinases following Dasatinib treatment. ZNF112 expresses ZMYM2-FGFR1, CEP2A and CEP5A cells express CEP110-FGFR1 and BBC1 expresses BCR-FGFR1. (B) Apoptosis and cell cycle analyses show that Dasatinib treatment (300 nM for ZNF112, CEP2A and CEP5A; 1000 nM for BBC, treated for 48h) remarkably increased cell apoptosis rate and decreased the percentage of cells in the S+G2 phase in ZMYM2-FGFR1 and CEP110-FGFR1 expressing cells. (C) Immunoprecipitation with anti-FRS2 in CEP2A and ZNF112 cells shows that both the phopho-FGFR1 fusion kinase and phospho-Src are present in the same immunocomplex. (D) Flow cytometry analysis shows infection with a dominant negative K295R/Y527F Src (pDNSrc) retroviral construct induces cell death in the cell lines expressing the chimeric FGFR1 kinases compared with the empty vector alone.
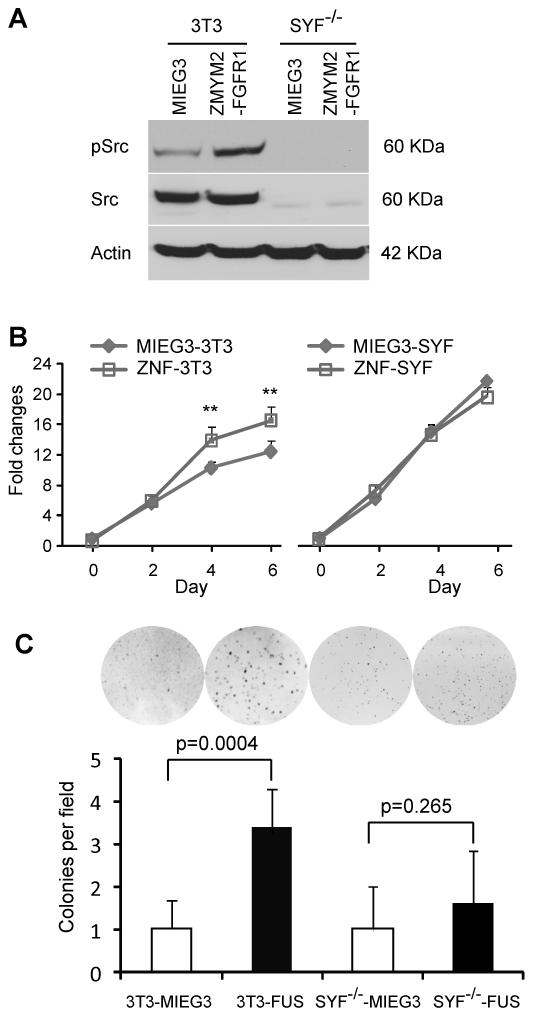
Knockout of Src impairs transformation of 3T3 cells by ZMYM2-FGFR1
Although Dasatinib inhibits both BCR/ABL and Src family tyrosine kinases, it can also inhibit other tyrosine kinases, such as PDGFRa/b and KIT (27-28). To demonstrate a specific association between FGFR1 chimeric kinase expression and Src activation, we introduced either the ZMYM2-FGFR1, or MIEG3, retroviral constructs into the Src kinase family null Src-Yes-Fyn (SYF) mouse embryo fibroblasts (MEF) (29). Non tumorigenic NIH3T3 cells were used as controls. Stably infected, GFP positive MEFs were isolated by FACS (Supplementary Fig. S1A). Western blot analysis showed that expression of ZMYM2-FGFR1 results in high levels of Src activation in normal NIH3T3 cells but not in the SYF−/− MEFs (Fig. 5A). Red fluorescent PKH26 was used to analyse cell cycle progression which demonstrated that ZMYM2-FGFR1 can significantly promote cell proliferation in NIH3T cells but not in SYF−/− MEFs (Supplementary Fig. S1B), which is consistent with activation of Src. To further support this observation, cell proliferation assays clearly show that ZMYM2-FGFR1 transformed 3T3 cell grow faster than cells transfected with the empty vector after 4-days. No difference was observed between ZMYM2-FGFR1 and MIEG3 transduced SYF cells (Fig 5B). Furthermore, Soft-agar colony formation demonstrated the development of larger colonies by the ZMYM2-FGFR1 infected NIH3T3 cells, compared with cells carrying the empty MIEG3 vector (Fig. 5C), although no difference was observed between ZMYM2-FGFR1 and MIEG3 infected MEF-SYF−/− cells (Fig. 5C). These results demonstrate that abrogation of Src activity can significantly reduce in vitro phenotypes of transformation in ZMYM2-FGFR1 expressing cells.
Figure 5. Knockdown of Src impairs transformation of 3T3 cells by ZMYM2-FGFR1.
(A) Src-Yes-Fyn null mouse embryo fibroblasts (SYF−/−) and NIH3T3 (3T3) cells were infected with ZMYM2-FGFR1 or MIEG3 retroviral constructs. Stably infected pools were sorted for GFP positive cells and then analyzed by western blotting with anti-phospho-Src antibodies. The phospho-Src levels are increased in ZMYM2-FGFR1 transformed 3T3 cells. In contrast, total and phospho Src proteins are not detectable in triple knockout SYF−/− cells transduced with ZMYM2-FGFR1. B) Cell proliferation assays demonstrate that ZMYM2-FGFR1 expression increases cell proliferation in NIH3T3, but not in SYF−/− cells. C) Transduction with ZMYM2-FGFR1 significantly promotes anchorage-independent growth of 3T3 cells (3T3-FUS) but not SYF−/− cells (SYF−/−-FUS).
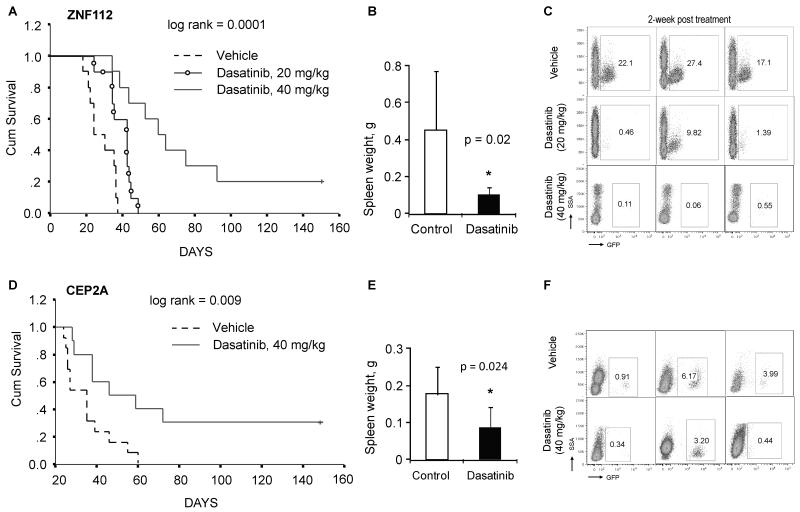
Dasatinib significantly reduces leukemogenesis
Src activation clearly plays a role in the in vitro tumorigenic potential of FGFR1 fusion kinases. To determine whether the same was true in vivo, we transplanted ZNF112 and CEP2A cell lines into normal female Balb/c mice and treated them with Dasatinib. Leukemogenesis was then monitored over several months. Our pilot studies demonstrated that both of these cell lines predominantly induce T-cell leukemia/lymphoma in vivo. Although injection of 100,000 cells from either cell line typically results in SCLL within 3 weeks, for the drug treatment experiments we injected 2 million cells into each mouse to ensure a large initial tumor burden. Dasatinib treatments were initiated 8 days after transplantation, to provide sufficient time for the injected cells to home to the bone marrow. Initially we treated ZNF112 carrying mice with Dasatinib at 20 mg per kg body weight, according to the regimen described by Hu et al. (18). In these experiments, Dasatinib prolonged the median survival time by 2 weeks (p < 0.05, Fig. 6A). We did not observe any remarkable side effects due to Dasatinib treatment in these mice, such as diarrhea or reduced body weight. Post mortem analyses did not reveal evidence of gastrointestinal hemorrhage, which is found in ~8% of human patients treated with Dasatinib (30). When we increased the Dasatinib dose to 40 mg/kg body weight in subsequent experiments, leukemogenesis was significantly inhibited for both ZNF112 and CEP2A-injected mice compared with animals treated with the vehicle control (Fig. 6A and D). 2/10 mice (20%) injected with ZNF112 cells and 30% (3/10) of mice injected with CEP2A show no GFP+ cells in their peripheral blood 4 months after transplantation (data not shown). Consistent with the survival analysis, the spleen weights in the Dasatinib-treated groups were also significantly decreased compared with control groups (Fig. 6B and E). Flow cytometric analysis of these Dasatinib-treated animals shows a remarkable decrease in GFP+CD4+CD8+ cells in the peripheral blood after only two weeks of treatment (Fig. 6C and F). Together, these data suggest that inhibition of Src activation or possibly other non-Src targets of Dasatinib can inhibit leukemogenesis induced by FGFR1 fusion kinases.
Figure 6. Dasatinib reduces lymphomogenesis of ZNF112 and CEP2A cells.
(A) ZNF112 xenografted into Balb/c mice (n=10) and then treated with DMSO (vehicle) rapidly succumb. Xenografted mice treated with 20mg/kg (n=10) Dasatinib prolongs survival and at 40mg/kg (n=10) significantly prolongs survival. In these animals (B), spleen enlargement in the 40mg/kg group, are significantly reduced. Two weeks after treatment using both doses shows reduced numbers of GFP+ cells in the peripheral blood of Dasatinib treated mice (C). In the same way, (D) treatment of CEP2A xenografts (n=10) with 40mg/kg Dasatinib significantly prolongs survival and reduces spleen weight (E) in these animals. Reduced numbers of GFP+ cells is also seen in this model (F).
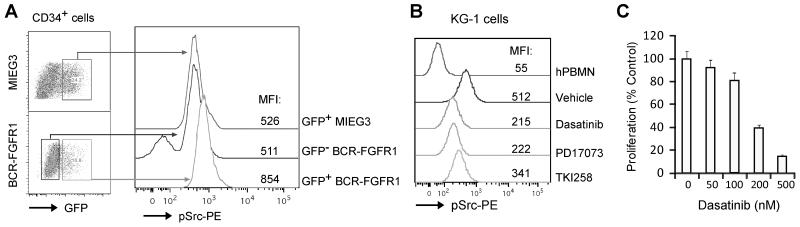
Activation of Src is also seen in BCR-FGFR1 transduced human CD34+ cells and the KG-1 cell line
To determine whether Src is activated in human hematopoietic cells, we transduced BCR-FGFR1 into CD34+ progenitor cells isolated from human cord blood samples. Intracellular phospho-flow cytometry analysis demonstrated that phopho-Src levels were increased 62% in the GFP+BCR-FGFR1 subpopulation compared with that in CD34+ cells transduced with the control vector (GFP+MIEG3 subpopulation) and increased 67% compared to normal CD34+ cells (GFP negative BCR-FGFR1) based on the mean of fluorescent intensity (MFI, Fig. 7A). This experiment suggested that FGFR1 fusion kinase could also induce Src activation in human hematopoietic progenitor cells. In addition, higher activated Src levels were also found in human KG-1 cells, expressing the FGFR1OP2-FGFR1 variant fusion gene (20), compared with the normal human mononuclear cells (Fig. 7B). Furthermore, when KG-1 cells were treated with either Dasatinib or the FGFR1 inhibitors PD17073 or TKI258, phospho-Src levels were reduced 1.5-2 fold compared with the vehicle treated cells (Fig. 7B), suggesting activation of Src in KG-1 cells could be associated with constitutive activation of FGFR1 fusion kinase. Treatment with Dasatinib at low nanomolar concentrations also induces growth inhibition in KG-1 cells (Fig. 7C), indicating Src activation plays an important role in sustaining KG-1 survival and proliferation. Together, these observations demonstrate that activation of Src is one of the mechanisms that drives chimeric FGFR1-associated tumorigenesis and progression. Consequently, molecularly targeting Src kinase may provide a new therapeutic option for SCLL patients.
Figure 7. Constitutive Src activation in BCR-FGFR1 transduced human CD34+ and KG-1 cells.
(A) Phospho-flow cytometry analysis showed that phospho-Src levels in BCR-FGFR1 transduced normal human CD34+ progenitor cells (GFP+BCR-FGFR1) is increased 62% compared with MIEG3 transduced CD34+ cells (GFP+MIEG3) based on the mean of florescent intensity (MFI) of the pSrc-PE channel. This experiment was repeated independently in triplicate and consistent results were obtained. (B) KG-1 cells, expressing FGFR1OP2-FGFR1, contain higher levels of phospho-Src compared with normal human mononuclear cells (hPBMN). Treatment with Dasatinib (100 nM), or FGFR1 inhibitors PD17073 (50 nM) or TKI258 (200 nM), inhibited Src activation in KG-1 cells. (C) Treatment with Dasatinib induces growth inhibition in KG-1 cells.
Discussion
SCLL is a distinct disease with bilineage involvement in the same patient. To date, a wide variety of therapeutic regimens have been used for patients with SCLL, including protocols for acute lymphoblastic leukemia, acute myeloid leukemia, chronic myelogenous leukemia and for other myeloproliferative neoplasms or myelodysplastic syndromes (6). Overall, the success of targeted therapy has not been achieved in patients with SCLL. In the present study we have demonstrated that activation of Src kinase is consistently seen in vitro in cells transformed with different chimeric FGFR1 kinases, as well as in vivo in mice transduced and transplanted with FGFR1 fusion genes. Pharmacological inhibition of Src kinase, using Dasatinib treatment or genetic inactivation of Src using dominant/negative construct, reduces cell proliferation in chimeric FGFR1 transformed cells. Dasatinib can also significantly delay leukemogenesis in vivo, and in 20-30% of cases can completely prevent tumorigenesis. These results provide proof-of-principle evidence that molecular targeting of Src kinase could be a potential strategy to treat SCLL patients, either alone, or in combination with other drugs.
Src overexpression and activation is found in a large number of human malignancies, including lung, breast, pancreatic, colon, and prostate (see review (31-32)). High level activation of Src has also been linked to the development of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia (33). In normal cells, Src plays a critical role in mediating signal transduction via interactions with multiple proteins and protein complexes, including the normal FGFR1 signaling pathway (15). Wild type FGFR1 is tethered in the cell membrane, while the ZMYM2-FGFR1 fusion protein, and other FGFR1 fusion molecules, are found in the cytoplasm due to loss of putative nuclear localization signals (34-35). Previously, it was assumed that the chimeric FGFR1 proteins had lost their ability to recruit Src kinase due to loss of the juxtamembrane FRS2 binding site in the chimeric protein (6, 19). However, we have now demonstrated that all FGFR1 fusion kinases retain part of the FRS2 (Fig. 1) which in IP experiments appears to be sufficient to bind FRS2 and lead to phospho-activaton of Src in both ZNF112 (harboring ZMYM2-FGFR1) and CEP2A (containing CEP110-FGFR1) cells. Consistent with these observations, Gu et al. demonstrated that 8/9 members of the Src family kinases were highly phosphorylated in the human KG-1 myeloid cell line, which harbors the rare variant FGFR1OP2-FGFR1 fusion kinase (20). These data indicate that activation of Src kinase may be a common event during initiation of FGFR1-related SCLL.
Dasatinib is a novel, potent, oral, multi-targeting inhibitor of Src, Abl1/2, KIT and PDGFRa/b at nanomolar levels (27-28). In the clinic, Dasatinib has shown efficacy in patients with imatinib-resistant CML, and currently Dasatinib is also the most effective treatment for patients with accelerated-phase CML (30). To date, Dasatinib has not apparently been used to treat SCLL patients. Here we show that Dasatinib treatment can substantially prolong the survival of mice with chimeric FGFR1-induced tumors. Consistent with these observations, we also demonstrated that the human KG-1 myeloid leukemic cells, which carry the FGFR1OP2-FGFR1 chimeric kinase, are also highly sensitive to Dasatinib treatment. These results provide preclinical evidence that Src inhibitors might be the basis for clinic trials involving SCLL patients. Our previous studies suggested that multiple genetic, and possibly epigenetic, changes are required for ZMYM2-FGFR1 to fully drive leukemogenesis and progression (17). We have recently identified activating deletions in the 5′ region of Notch1 in all T-ALL samples from ZMYM2-FGFR1 leukemia/lymphoma mice (36) which provides convincing evidence that ZMYM2-FGFR1 can drive the development of T-lineage disease through non-random constitutive activation of Notch1. Interestingly, aberrant activation of Notch1 was also seen in cells both from primary leukemic cells from a patient with ZMYM2-FGFR1 SCLL, as well as KG-1 cells. Although these observations demonstrate that targeting either Src or Notch1 alone in SCLL patients, may provide some benefit, targeting FGFR1, SRC and Notch1 in combination might be more effective.
Treatment of cells expressing ZMYM2-FGFR1 and CEP110-FGFR1 kinases with TK1258 reduced FGFR1 activation with a concomitant reduction in Src activation. However, TKI258 did not significantly inhibit Src activation in cells expressing the BCR-FGFR1 kinase (Fig. 1F). Furthermore, targeting Src with Dasatinib only induced apoptosis and death in vitro at high concentrations. Consistent with this observation, in vivo treatment of BCR-FGFR1-expressing cells with the same dosage of Dasatinib that prolonged survival in mice transplanted with ZMYM2-FGFR1 and CEP110-FGFR1 expressing cells, did not prolong survival in these BCR-FGFR1 mice (data not shown). SCLL patients expressing the BCR-FGFR1 kinase differ from SCLL patents expressing the other kinases, in that they present with an atypical chronic myeloid leukemia (CML), without the Philadelphia chromosome (3, 37). In mice, BCR-FGFR1 rapidly induces CML-like MPD and B-cell lymphoma, with significantly shortened survival times compared with ZMYM2-FGFR1 (19) and Tidwell et al. manuscript submitted), as well as in CEP110-FGFR1-induced disease (Ren, in preparation). Unlike the other fusion partners, BCR itself also has kinase activity (38) and so potentially serves not only as an oligomerization domain to activate FGFR1, but can also trigger downstream signaling pathways in its own right. Mutation of the critical Y177 tyrosine in BCR-FGFR1, which is required to bind the GRB2 adaptor protein, significantly reduces murine SCLL development in vivo (19) and those mice that develop disease show T-cell lymphomas typical of the other fusion kinases. Taken together, these observations suggest that the BCR component of the fusion kinase may be driving the disease to the myeloid and B-cell lineage and that targeting FGFR1 alone in this disease is not as efficient a therapeutic approach as it is in the other chimeric kinase-induced diseases. From our data, it appears that this may be due in some part to the inability of FGFR1 targeting drugs to specifically suppress Src activation.
In summary, our present study demonstrates that ZMYM2-FGFR1, BCR-FGFR1 and CEP110-FGFR1 chimeric proteins can constitutively activate Src kinase both in vitro and in vivo. Pharmacological and genetic inhibition of Src function induces apoptosis and cell death in cells harboring these chimeric FGFR1 genes suggesting that that Src activation is an important oncogenic event for FGFR1 fusion gene driven disease development and progression. As such, molecular targeting of Src kinase should be considered as part of a combination treatment for SCLL patients.
Supplementary Material
Acknowledgments
This work was supported by grant CA076167 from the National Institutes of Health. We thank Dr. Dario Vignali for providing the YFP pMIYII vector.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Vega F, Medeiros LJ, Davuluri R, Cromwell CC, Alkan S, Abruzzo LV. t(8;13)-positive bilineal lymphomas: report of 6 cases. Am J Surg Pathol. 2008;32:14–20. doi: 10.1097/PAS.0b013e31814b226e. [DOI] [PubMed] [Google Scholar]
- 2.Abruzzo LV, Jaffe ES, Cotelingam JD, Whang-Peng J, Del Duca V, Jr., Medeiros LJ. T-cell lymphoblastic lymphoma with eosinophilia associated with subsequent myeloid malignancy. Am J Surg Pathol. 1992;16:236–45. doi: 10.1097/00000478-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, et al. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–83. doi: 10.1182/blood.v98.13.3778. [DOI] [PubMed] [Google Scholar]
- 4.Guasch G, Ollendorff V, Borg JP, Birnbaum D, Pebusque MJ. 8p12 stem cell myeloproliferative disorder: the FOP-fibroblast growth factor receptor 1 fusion protein of the t(6;8) translocation induces cell survival mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt/mTOR pathways. Mol Cell Biol. 2001;21:8129–42. doi: 10.1128/MCB.21.23.8129-8142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao S, McCarthy JG, Aster JC, Fletcher JA. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood. 2000;96:699–704. [PubMed] [Google Scholar]
- 6.Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Wasag B, Lierman E, Meeus P, Cools J, Vandenberghe P. The kinase inhibitor TKI258 is active against the novel CUX1-FGFR1 fusion detected in a patient with T-lymphoblastic leukemia/lymphoma and t(7;8)(q22;p11) Haematologica. 2011;96:922–5. doi: 10.3324/haematol.2010.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuskonmaz B, Kafali C, Akcoren Z, Karabulut HG, Akalin I, Tuncer MA. The 8p11 myeloproliferative syndrome in a 3-year-old child. Leuk Res. 2008;32:198–9. doi: 10.1016/j.leukres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Suzan F, Guasch G, Terre C, Garcia I, Bastie JN, Maarek O, et al. Long-term complete haematological and molecular remission after allogeneic bone marrow transplantation in a patient with a stem cell myeloproliferative disorder associated with t(8;13)(p12;q12) Br J Haematol. 2003;121:312–4. doi: 10.1046/j.1365-2141.2003.04269.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong WS, Cheng KC, Lau KM, Chan NP, Shing MM, Cheng SH, et al. Clonal evolution of 8p11 stem cell syndrome in a 14-year-old Chinese boy: a review of literature of t(8;13) associated myeloproliferative diseases. Leuk Res. 2007;31:235–8. doi: 10.1016/j.leukres.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007;6:550–66. doi: 10.4161/cc.6.5.3919. [DOI] [PubMed] [Google Scholar]
- 12.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 13.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–50. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 14.Kilkenny DM, Rocheleau JV, Price J, Reich MB, Miller GG. c-Src regulation of fibroblast growth factor-induced proliferation in murine embryonic fibroblasts. J Biol Chem. 2003;278:17448–54. doi: 10.1074/jbc.M209698200. [DOI] [PubMed] [Google Scholar]
- 15.Sandilands E, Akbarzadeh S, Vecchione A, McEwan DG, Frame MC, Heath JK. Src kinase modulates the activation, transport and signalling dynamics of fibroblast growth factor receptors. EMBO Rep. 2007;8:1162–9. doi: 10.1038/sj.embor.7401097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson H, Klint P, Landgren E, Claesson-Welsh L. Fibroblast growth factor receptor-1-mediated endothelial cell proliferation is dependent on the Src homology (SH) 2/SH3 domain-containing adaptor protein Crk. J Biol Chem. 1999;274:25726–34. doi: 10.1074/jbc.274.36.25726. [DOI] [PubMed] [Google Scholar]
- 17.Ren M, Li X, Cowell JK. Genetic fingerprinting of the development and progression of T-cell lymphoma in a murine model of atypical myeloproliferative disorder initiated by the ZNF198-fibroblast growth factor receptor-1 chimeric tyrosine kinase. Blood. 2009;114:1576–84. doi: 10.1182/blood-2009-03-212704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–5. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, Cross NC, et al. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–98. doi: 10.1016/s1535-6108(04)00053-4. [DOI] [PubMed] [Google Scholar]
- 20.Gu TL, Goss VL, Reeves C, Popova L, Nardone J, Macneill J, et al. Phosphotyrosine profiling identifies the KG-1 cell line as a model for the study of FGFR1 fusions in acute myeloid leukemia. Blood. 2006;108:4202–4. doi: 10.1182/blood-2006-06-026666. [DOI] [PubMed] [Google Scholar]
- 21.Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, et al. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–89. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Lee KW, Goldfarb M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J Biol Chem. 1998;273:17987–90. doi: 10.1074/jbc.273.29.17987. [DOI] [PubMed] [Google Scholar]
- 23.Kasyapa CS, Kunapuli P, Hawthorn L, Cowell JK. Induction of the plasminogen activator inhibitor-2 in cells expressing the ZNF198/FGFR1 fusion kinase that is involved in atypical myeloproliferative disease. Blood. 2006;107:3693–9. doi: 10.1182/blood-2005-04-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetzler M, Brady MT, Tracy E, Li ZR, Donohue KA, O’Loughlin KL, et al. Arsenic trioxide affects signal transducer and activator of transcription proteins through alteration of protein tyrosine kinase phosphorylation. Clin Cancer Res. 2006;12:6817–25. doi: 10.1158/1078-0432.CCR-06-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110:3729–34. doi: 10.1182/blood-2007-02-074286. [DOI] [PubMed] [Google Scholar]
- 26.Bagrodia S, Chackalaparampil I, Kmiecik TE, Shalloway D. Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature. 1991;349:172–5. doi: 10.1038/349172a0. [DOI] [PubMed] [Google Scholar]
- 27.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 28.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 29.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–71. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantarjian H, Cortes J, Kim DW, Dorlhiac-Llacer P, Pasquini R, DiPersio J, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–9. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 32.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 33.Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–61. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- 34.Ollendorff V, Guasch G, Isnardon D, Galindo R, Birnbaum D, Pebusque MJ. Characterization of FIM-FGFR1, the fusion product of the myeloproliferative disorder-associated t(8;13) translocation. J Biol Chem. 1999;274:26922–30. doi: 10.1074/jbc.274.38.26922. [DOI] [PubMed] [Google Scholar]
- 35.Kunapuli P, Kasyapa CS, Chin SF, Caldas C, Cowell JK. ZNF198, a zinc finger protein rearranged in myeloproliferative disease, localizes to the PML nuclear bodies and interacts with SUMO-1 and PML. Exp Cell Res. 2006;312:3739–51. doi: 10.1016/j.yexcr.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Ren M, Cowell JK. Constitutive Notch pathway activation in murine ZMYM2-FGFR1-induced T-cell lymphomas associated with atypical myeloproliferative disease. Blood. 2011;117:6837–47. doi: 10.1182/blood-2010-07-295725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fioretos T, Panagopoulos I, Lassen C, Swedin A, Billstrom R, Isaksson M, et al. Fusion of the BCR and the fibroblast growth factor receptor-1 (FGFR1) genes as a result of t(8;22)(p11;q11) in a myeloproliferative disorder: the first fusion gene involving BCR but not ABL. Genes Chromosomes Cancer. 2001;32:302–10. doi: 10.1002/gcc.1195. [DOI] [PubMed] [Google Scholar]
- 38.Maru Y, Witte ON. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991;67:459–68. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.