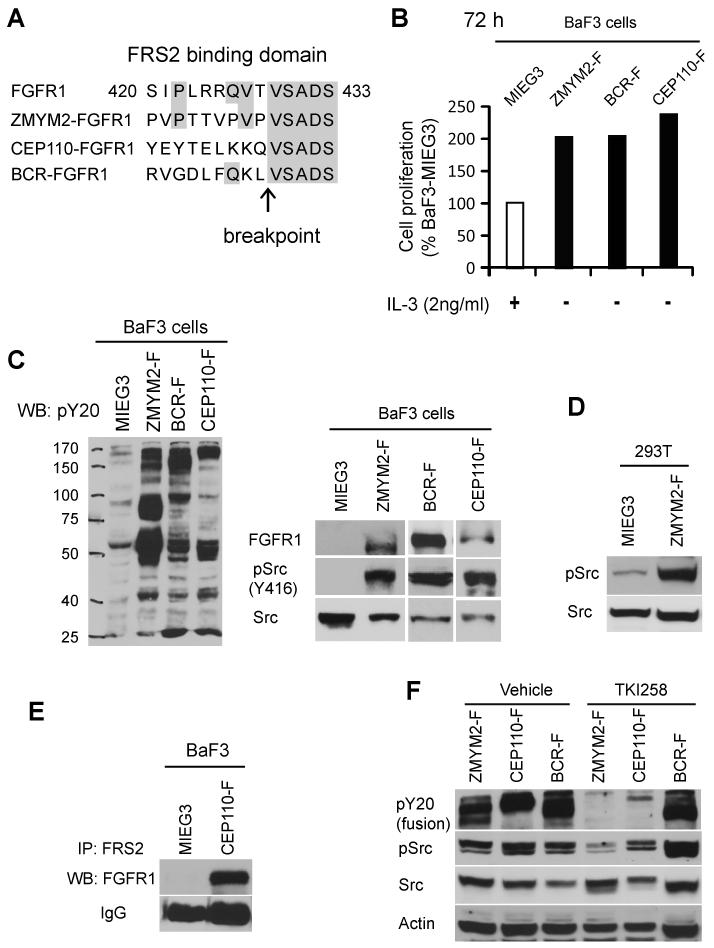
Figure 1. Activation of Src is associated with FGFR1 chimeric kinese expression.
A) Schematic outline of the FGFR1 break point junction in the chimeric FGFR1 fusion kinases shows conserved amino acids within the FRS2 consensus binding domain (amino acids 420-433) (21-22). (B) Cell proliferation rates in BaF3 cells transformed by ZMYM2-FGFR1 (ZMYM2-F), BCR-FGFR1 (BCR-F) or CEP110-FGFR1 (CEP110-F) show both IL-3 independence and an increased growth rate relative to cells carrying the empty vector (MIEGS). (C) Transduction of BaF3 cells (left panel) with the chimeric FGFR1 kinases identifies constitutive tyrosine phosphorylation in total cellular proteins using the anti-PY20 antibody compared with cells carrying the empty vector. These cells also show phospho-activation of Src kinase (right panel). (D) Ectopic expression of ZMYM2-FGFR1 in adherent 293T cells also leads to increased levels of phospho-Src. (E) Immunoprecipitation using anti-FRS2 antibodies co-precipitates the CEP110-FGFR1 fusion protein in CEP110-FGFR1 (CEP110-F) transformed BaF3 cells, but not in cells transduced with the empty vector. (F) When BaF3 cells expressing either ZMYM2-FGFR1 or CEP110-FGFR1 are treated with the TKI258 (400 nM) FGFR1 inhibitor, activation of both the FGFR1 fusion kinases and Src is inhibited. In contrast, TKI258 does not affect activation of either BCR-FGFR1 or Src in BCR-FGFR1 transformed cells.