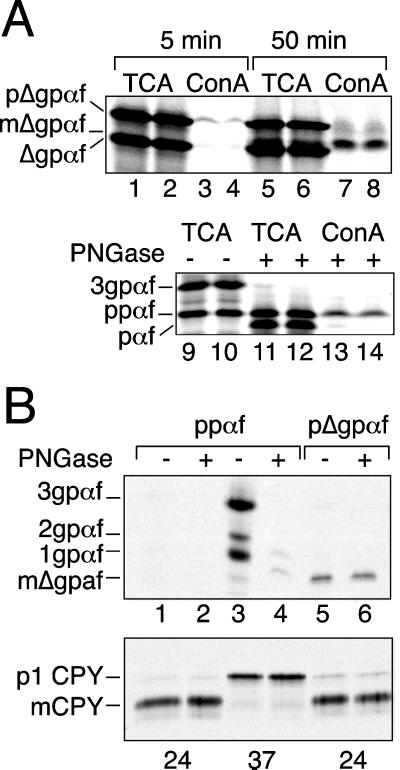
Figure 1.
Mutant alpha-factor precursor is O-mannosylated in the ER. (A) Prolonged ER residence of Δgpαf in vitro results in O-mannosylation. In vitro translated, [35S]methionine-labeled pΔgpαf (top) or ppαf (bottom) was translocated into wild-type yeast microsomes at 24°C in the presence of ATP and an ATP-regenerating system for the indicated periods of time. Duplicate samples were either TCA precipitated or membranes lysed and proteins precipitated directly with ConA-Sepharose (lanes 3, 4, 7, and 8) or first treated with PNGase and then precipitated with ConA-Sepharose as indicated (lanes 13 and 14). Proteins were analyzed by SDS-PAGE on 18% 4 M urea gels and autoradiography. Note that the nonspecific binding of ppαf to ConA-Sepharose is higher than that of pΔgpαf; this is an intrinsic property of ppαf. (B) Δgpαf is O-mannosylated in vivo. RSY281 (sec23 ts) was labeled with [3H]mannose for 90 min at the permissive (24°C) or at the restrictive (37°C) temperature. Cells were lysed, and CPY and alpha-factor precursor were immunoprecipitated. Alpha-factor immunoprecipitates shown in lanes 2, 4, and 6 were PNGase digested before SDS-PAGE and autoradiography. Note that a small amount of 1gpαf in lane 4 was refractory to PNGase digestion.