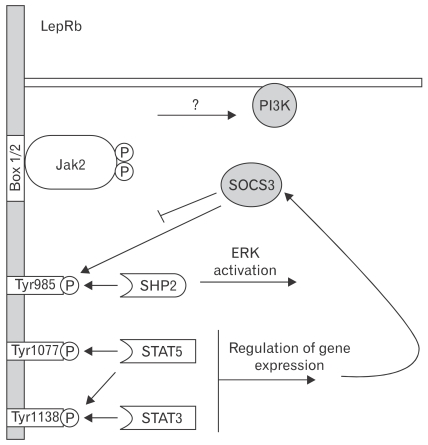
Figure 2.
The role of discrete leptin receptor b (LepRb) functional sites in leptin signaling. Leptin binding to LepRb activates the associated Janus kinase-2 (Jak2) tyrosine kinase bound at the Box1/2 motifs. Activated Jak2 undergoes robust autophosphorylation and phosphorylates Tyr985, Tyr1077 and Tyr1138 on the LepRb intracellular tail. These phosphorylated residues act as docking sites for SH2-domain containing proteins. Phosphorylated Tyr985 mediates docking with SH2 domain-containing tyrosine phosphatase 2 and subsequent activation of extracellular signal-regulated kinase through the mictogen-activated protein kinase signaling cascade. Phosphorylated Tyr1077 mediates signal transducer and activator of transcription 5 (STAT5) activation. Phosphorylated Tyr1138mediates both STAT3 and STAT5 activation. STAT3 activation ultimately leads to increased expression of suppressor of cytokine signaling-3, which acts as a feedback inhibitor and negatively regulates LepRb signaling in part by binding phosphorylated Tyr985. Leptin also activates phosphoinositide 3-kinase, although the intermediated steps for this process remain obscure. PI3K, phosphoinositide 3-kinase; SOCS3, suppressor of cytokine signaling-3; SHP2, SH2 domain-containing tyrosine phosphatase 2; ERK, extracellular signal-regulated kinase. Modified figure adopted from Robertson et al64 with permission from Elsevier.