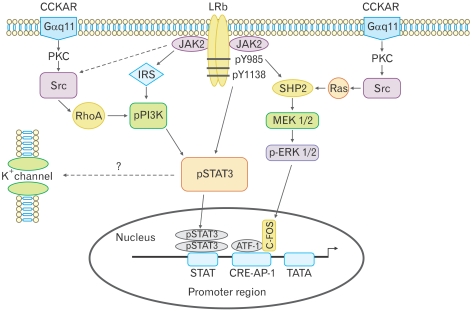
Figure 4.
Proposed signal transduction pathways in nodose ganglia following receptor activation with leptin and cholecystokinin-8 (CCK-8). There are 2 potential pathways for phosphorylation of signal transducer and activator of transcription 3 (STAT3). Leptin activates Janus kinase-2 phosphorylation at tyrosine 1138, which directly phosphorylates STAT3, or leptin activates phophoinositide 3-kinase (PI3K) via the insulin receptor substrate, leading to STAT3 phosphorylation. CCK-8 activates PI3K via SRC and RhoA, which leads to phosphorylation of STAT3, suggesting that PI3K is central to the synergistic leptin/CCK STAT3 phosphorylation. The mitogen-activated protein kinase (MAPK) inhibitor PD98059 had no effect on leptin and CCK-8 synergism, suggesting that the leptin/CCK-8-stimulated MAPK/ extracellular signal-regulated kinase 1/2 pathway was not involved in STAT3 phosphorylation. STAT3 usually acts by stimulating the transcription of target genes, but the rapid electrophysiological effects suggest STAT3 may be involved in modifying the activity of K+ channels. CCKAR, CCK-A receptor; LRb, leptin receptor b; PKC, protein kinase C; JAK2, Janus kinase-2; IRS, insulin receptor substrate; SHP2, SH2 domain-containing tyrosine phosphatase 2; ERK, extracellular signal-regulated kinase; MEK, MAPK/ERK kinase; ATF-1, activating transcription factor-1; CRE, cAMP response element-binding protein; AP-1, activator protein-1. Adapted from Heldsinger et al.14