Table 1.
 | |||
|---|---|---|---|
| entry | metal salt | metal salt/ KF equiv |
yield (%) |
| 1 | AgOAc | 2/2 | 6 |
| 2 | Ag2O | 2/2 | 6 |
| 3 | AgNO3 | 2/2 | 40 |
| 4 | AgF | 2/2 | 45 |
| 5 | AgOTf | 2/2 | 68 |
| 6 | AgOTf | 4/4 | 87 |
| 7 [c] | AgOTf | 4/0 | 0 |
| 8 [d] | AgOTf | 4/4 | 53 |
| 9 | [CuOTf]2·C6H6 | 2/2 | 0 |
| 10 | CuI | 4/4 | 0 |
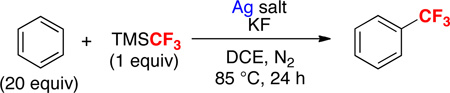
General conditions: C6H6 (20 equiv), TMSCF3 (1 equiv) in DCE at 85 °C for 24 h.
Yields determined by 19F NMR analysis.
No KF.
Conditions: C6H6 (1 equiv), TMSCF3 (5 equiv), AgOTf (4 equiv), KF (4 equiv) in DCE at 85 °C for 24 h.
