Figure 7.
CRL3NPH3 Function Is Required for Ubiquitination and Degradation of phot1.
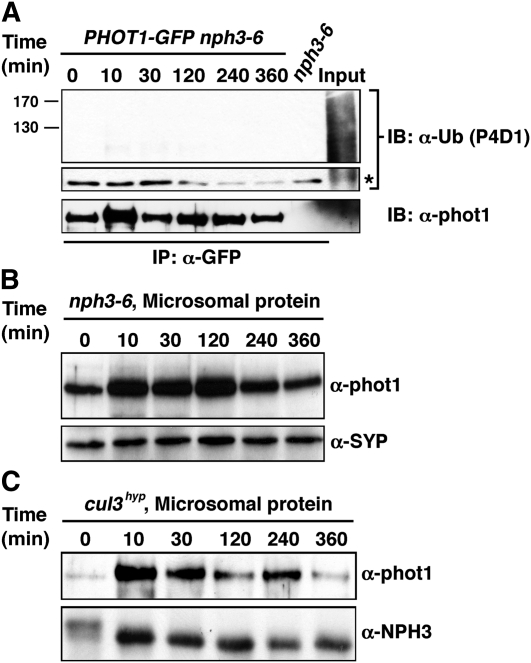
(A) High-intensity BL-dependent ubiquitination of phot1 is absent in the nph3-null mutant background. Total cellular proteins were prepared from Arabidopsis phot1-5nph3-6PHOT1-GFP seedlings that had been mock irradiated (0 min) or exposed to high-intensity unilateral BL (120 μmol m−2 s−1) for the indicated times (lanes 1 to 6). Total cellular proteins were also prepared from mock irradiated nph3-6 seedlings as a negative control (lane 7). With the exception of aliquots from the phot1-5PHOT1-GFP samples that were retained as positive controls for total cellular ubiquitination (Input, lane 8), all samples were subjected to immunoprecipitation (IP) with anti-GFP antibodies (lanes 1 to 7). In the top two panels, ubiquitinated proteins were detected by immunoblot (IB) analysis with P4D1 anti-Ub antibodies. Middle panel shows an extracted portion of the same blot from the top panel containing IgG heavy chain (~50 kD) from the α-GFP antibody (asterisk) as a loading control for IP samples. Bottom panel shows a replicate blot probed with anti-phot1 antibodies to demonstrate that although ubiquitinated phot1 is not detected by the P4D1 antibody (top panel), phot1-GFP is in fact immunoprecipitated effectively from phot1-5nph3-6PHOT1-GFP seedlings grown under high BL conditions.
(B) BL fails to stimulate phot1 degradation in the nph3-null mutant background. Microsomal proteins were prepared from nph3-6 seedlings, and phot1 was detected by immunoblot (IB) analysis with anti-phot1 antibodies (top panel). Bottom panel shows a replicate blot probed with an anti-syntaxin antibody (α-SYP) as a plasma membrane–specific protein loading control.
(C) BL-induced degradation of phot1 is retarded in the cul3hyp mutant background. Microsomal proteins were prepared from cul3hyp seedlings and phot1 detected in the top panel as described in (B). Bottom panel shows a replicate blot probed with an anti-NPH3 antibody as a plasma membrane–specific protein loading control.