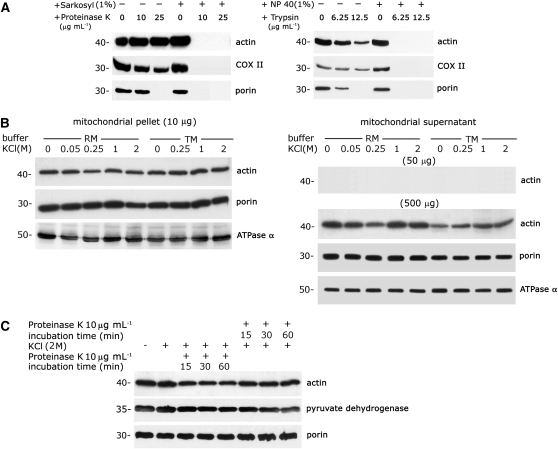
Figure 1.
A Large Portion of Mitochondria-Localized Actin Is Resistant to Protease and High-Salt Treatments.
(A) A marked proportion of actin is localized to proteinase K– and trypsin-treated mitochondria. Purified mitochondria (5 μg protein) from 3-d-old mung bean seedlings were treated with different concentrations of proteinase K (left section) and trypsin (right section) with or without prelysing with detergent (1% [w/v] sarkosyl or 1% [w/v] Nonidet P-40). Immunoblot analyses with antiactin, anti-COX II (inner membrane protein), and antiporin (outer membrane protein) antibodies were performed after gel transfer.
(B) A large proportion of actin associated with mitochondria is not loosely bound or peripherally bound. Purified mitochondria were treated with various concentrations of KCl (0 to 2 M) in two different buffers (RM and TM). After two consecutive high-speed centrifugations and washing, the stripped mitochondria in the pellet and the dissociated proteins in the supernatant were collected and subjected to immunoblot analysis. Detection of actin in the supernatant was performed with fivefold (50 μg) and 50-fold (500 μg) excesses of protein (referring to the protein in the initial mitochondrial sample) by immunoblot analysis. Immunoblot analysis was performed with antibodies against actin, ATPase α (an inner membrane protein), and porin (an outer membrane protein).
(C) A large fraction of mitochondria-localized actin is resistant to a combined treatment with high salt and proteinase K. Purified mitochondria were subjected to progressive proteolytic digestion either before or after high-salt treatment and were repurified by centrifugation through a sucrose cushion. Antibodies against actin, pyruvate dehydrogenase (a matrix protein), and porin (an outer membrane protein) were used for immunoblot analysis.
Four independent experiments were performed for the above studies. Equivalent results were obtained, and the results of one experiment are presented here.