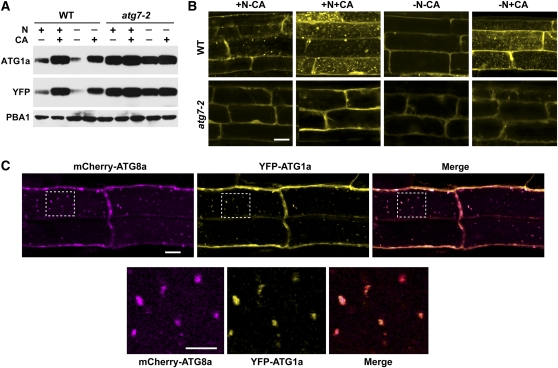
Figure 10.
ATG1a Associates in the Vacuole with ATG8a-Decorated Autophagic Bodies.
(A) Levels of YFP-ATG1a protein are reduced by N starvation–induced autophagy. Wild-type (WT) and atg7-2 seedlings were grown for 6 d on N-rich agar medium and then transferred to N-rich (+N) or N-deficient (–N) liquid media without or with 0.5 μM CA (+CA) for 16 h. YFP-ATG1a protein levels were measured by immunoblots with anti-ATG1a and anti-YFP antibodies. Immunoblot analysis with anti-PBA1 antibodies was used to confirm equal protein loading.
(B) YFP-ATG1a associates with autophagic bodies in a process that requires ATG7. Seven-day-old wild-type and atg7-2 seedlings were exposed to N-containing or N-deficient liquid media with or without 0.5 μM CA. After 16 h, the root tip cells were imaged by fluorescence confocal microscopy. Bar = 10 μm.
(C) YFP-ATG1a and mCherry-ATG8a colocalize in autophagic bodies. Seven-day-old wild-type plants expressing both reporters were grown on +Suc−N medium and then treated for 8 h with 0.5 μM CA. Root tip cells were imaged by fluorescence confocal microscopy for YFP and mCherry. Merged represents the superimposition of the two images. Bar = 10 μm. Higher magnifications of the vacuolar lumen (outline by dashed lines) demonstrating colocalization of the two proteins on autophagic bodies are shown below. Bar = 5 μm.