Abstract
Objective
To evaluate the association between early hypocarbia and 18-22 month outcome among neonates with hypoxic-ischemic encephalopathy (HIE).
Study design
Data from the NICHD NRN randomized controlled trial of whole body hypothermia for neonatal HIE were used for this secondary observational study. Infants (n=204) had multiple blood gases recorded from birth-12h of study intervention (hypothermia vs. intensive care alone). The relationship between hypocarbia and outcome (death/disability at 18-22 months) was evaluated by unadjusted and adjusted analyses examining minimum PCO2 and cumulative exposure to PCO2 <35 mmHg. The relationship between cumulative PCO2 <35 mmHg (calculated as the difference between 35mmHg and the sampled PCO2 multiplied by the duration of time spent <35 mmHg) and outcome was evaluated by level of exposure (none-high) using a multiple logistic regression analysis with adjustments for pH, level of encephalopathy, treatment group (± hypothermia), time to spontaneous respiration and ventilator days; results were expressed as OR and 95% confidence intervals. Alternative models of CO2 concentration were explored to account for fluctuations in CO2.
Results
Both minimum PCO2 and cumulative PCO2 <35mmHg were associated with poor outcome (P<0.05). Moreover, death/disability increased with greater cumulative exposure to PCO2 <35mmHg.
Conclusion
Hypocarbia is associated with poor outcome following HIE.
Keywords: hypocarbia, hypoxic ischemic encephalopathy, whole body hypothermia, outcome, neurodevelopmental impairment
Both pH and PCO2 affect vascular tone, cerebral blood flow, cerebral oxygenation1 and may thereby modulate neuronal injury in neonates with hypoxic-ischemic encephalopathy (HIE). Experimental evidence suggests that low PCO2 concentrations mediated by hyperventilation in brain injured patients may restore cerebral autoregulation2 and compensate for metabolic acidosis thus preventing further damage. Yet hypocarbia also reportedly contributes to detrimental effects: cerebral vasoconstriction, decreased partial pressure of arterial oxygen, decreased oxygen release from hemoglobin1,3 and excessive neuronal excitability due to increased oxygen demands4. Moreover, animal models of hypocarbia demonstrate nuclear DNA fragmentation5,6, decreased levels of high energy phosphates as well as neuronal7 and mitochondrial8 alterations that lead to apoptotic cell death. In the preterm infant, hypocarbia has been associated with periventricular leukomalacia9,10, cerebral palsy and neurodevelopmental deficits11. Klinger et al reported that episodic hyperoxia and hypocarbia within the first two hours of life are associated with an increased risk of brain injury after HIE12. They postulated that aggressive early management and resuscitation may be contributory. Hypocarbia, in the context of HIE, also may reflect the severity of neural injury (decreased CO2 production) or the infant’s own respiratory drive and ability to correct metabolic acidosis. Moreover, hypocarbia may be impacted by cooling13; hypothermia decreases the rate of brain energy utilization by 5.3% for every 1°C reduction in brain temperature below 38.2°C14 and decreases the basal metabolic rate by 25-30% at 33°C15. So the initial ventilator minute volume required to maintain normocarbia may be significantly lower for infants undergoing whole body cooling. We hypothesized that infants exposed to early hypocarbia following HIE may be at increased risk for death or disability. The present study examines the association between isolated severe hypocarbia (minimum PCO2) and cumulative exposure to PCO2 <35mmHg in the first 16 hours of life and adverse 18-22 month outcome (death or moderate to severe disability) among the participants of the NICHD Neonatal Research Network trial of whole body cooling for neonatal HIE16.
METHODS
This is a secondary study to the NICHD randomized trial of whole body cooling16 in encephalopathic ≥ 36 weeks gestational age infants admitted to the hospital within 6 hours of life with either severe acidosis or perinatal complications and resuscitation at birth. The study was performed in the participating centers of the Eunice Kennedy Shriver NICHD Neonatal Research Network and was approved by the Institutional Review Board of each of the participating centers. Written informed consent was obtained from the parents or guardians of each of the participants.
The infants had multiple blood gases recorded prospectively: pre-randomization, randomization, four, eight and twelve hours of intervention. Subsequent blood gases were obtained as per clinical care and were recorded once daily during study intervention. The blood gases were temperature corrected to 37°C via the blood gas analyzers for the hypothermia group. During hypothermia, the pH increases and the partial pressure of CO2 decreases compared with measurements made at normal temperatures. So a pH of 7.4 and a pCO2 of 40.0 mmHg at 37°C in a healthy infant will correspond with a pH of 7.5 and a pCO2 of 34mmHg at 33°C13. Most studies on temperature correction of blood gases have been performed in hypothermic patients undergoing cardiopulmonary bypass surgery. With temperature correction (i.e., pH-stat management), mechanical ventilation and CO2 retention are externally controlled to maintain normal pH and pCO2 of the temperature corrected values. This management was adopted for this trial. Centers treated infants as per usual care at each site regarding further ventilator management; the protocol did not dictate other ventilatory parameters.
Minimum PCO2 and cumulative exposure to PCO2 <35 mmHg (calculated as the difference between 35 mmHg and the sampled PCO2 multiplied by the duration of time spent below 35 mmHg10) were the focus of this study. Cumulative exposure to hypocarbia was calculated based only on recorded blood gas values for the relevant time period; for the few cases of missing blood gas values, we did not interpolate the missing value from earlier or surrounding values as we felt it was unwarranted to extend the period of hypocarbia. Alternative measures of CO2 concentrations were examined to account for variability in CO2 concentrations over time. Fluctuations in PCO2 were addressed by calculating the difference between maximum and minimum PCO2 and SD of PCO217. Also, hypercarbia (maximum PCO2) and cumulative exposure to hypercarbia (the difference between the sampled PCO2 and 50 mmHg multiplied by the duration of time spent above 50 mmHg summed from birth-12h of intervention) were examined as hypercarbia has been independently associated with neurological outcome18,19.
The neurodevelopmental outcome of the study participants was assessed at 18 to 22 months of age and included data on growth, vision, audiometric testing and standardized neurodevelopmental assessments performed by trained examiners masked to hypocarbia and treatment group status16. The neurodevelopmental evaluation included an assessment of cerebral palsy, functional disability (according to the modified gross motor function classification system (GMFCS) of Palisano)20 and the Bayley Scales of Infant Development, second edition21. Disability was graded as moderate or severe. Severe disability was defined as any of the following: a Bayley Mental Developmental Index score more than two standard deviations below the mean (i.e., below 70), a GMFCS grade of level 3 to 5, hearing impairment requiring hearing aids or blindness (<20/200 vision). Moderate disability was defined as a Mental Developmental Index score one to two SD below the mean score (i.e., 70 to 84) in addition to one or more of the following: a GMFCS grade of level 2, hearing impairment with no amplification, or a persistent seizure disorder at the time of follow-up.
The maternal and neonatal clinical characteristics between infants with and without hypocarbia were compared by t-tests for continuous variables and chi-square tests for categorical variables. The unadjusted associations between minimum PCO2, maximum PCO2, difference between minimum and maximum PCO2, and SD of PCO2 and outcome (as described by Fabres et al 17) were analyzed using the Wilcoxon rank sum test. Multiple logistic regression analysis was used to evaluate the relationship between these variables of CO2 concentrations and death/disability. In addition, the relationship between cumulative exposure to moderate hypocarbia (PCO2 <35mmHg) and death/disability was evaluated as a 5-level variable (quartiles of lowest to highest exposure and none) using multiple logistic regression analysis, adjusting for the following variables: systemic pH at randomization, initial level of encephalopathy, treatment group (± hypothermia), time to spontaneous respiration >10 minutes and days on mechanical ventilation. Adjustment for pH was deemed important as this varied significantly between infants with and without hypocarbia; moreover, given the known relationship between systemic pH and PCO2 concentration, we wanted to make sure that hypocarbia was not merely a surrogate marker for more severe initial acidosis with respiratory compensation. We are aware that systemic pH is not a good indicator of brain stem pH which may regulate respiratory effort and subsequent PCO2. To control for treatment effects and the initial severity of injury, we also examined the following variables: treatment group (± hypothermia), center, initial level of encephalopathy, presence of clinical seizures, time to spontaneous respiration and days on mechanical ventilation. All variables significantly associated with outcome were included in the final multivariate model. Goodness-of-fit between models was compared by the area-under-the-curve (AUC) c-statistic. In addition, subgroup analyses were performed to assess the relationship between hypocarbia and adverse outcome for the hypothermia group and intensive care alone group separately. Also, subgroup analyses were performed to assess the interaction between hypocarbia and hyperoxia (PaO2 >100 mmHg or 200 mmHg) and the association between hyperoxia and death/disability.
RESULTS
The average age at randomization was 4.3 hours and the average age at cooling was five hours (i.e., 35.5 minutes after randomization). Detailed blood gas analyses were available during the first 12 hours of study intervention, corresponding to the first 16.9±2.2 hours of life (mean ± SD) for 204 of 208 infants. Primary outcome data were available for all 204 infants. The source of the blood gas was recorded for the first postnatal blood gas (150 arterial samples, 39 venous, 12 capillary and 3 unknown). The number of participants with outcome and blood gas data available at each point in time was 199 between birth and randomization, 201 at randomization, 180 at 4 hours, 180 at 8 hours and 173 at 12 hours. There was one early death for which the cumulative exposure to hypocarbia needed to be truncated.
The median PCO2 (and interquartile range (IQR)) of the first postnatal blood gas was 33 mmHg (IQR 27mmHg); it was 32 mmHg at randomization (IQR 12mmHg), 32 mmHg at 4 hours (IQR 14.5mmHg), 35 mmHg at 8 hours (IQR 12mmHg), and 34 mmHg at 12 hours of study intervention (IQR 12mmHg). 181 neonates had at least one PCO2 concentration below 35 mmHg, and 100 neonates had at least one PCO2 concentration below 25 mmHg from birth to 12 hours of intervention (~16.9 hours of age(mean)). The characteristics of infants with and without cumulative hypocarbia (<35 mmHg) are shown in Table I. Infants with hypocarbia >50th percentile had a significantly lower cord pH and birth weight and a significantly higher likelihood of seizures compared with infants with hypocarbia <50th percentile or no hypocarbia. In addition, infants with cumulative hypocarbia >50th percentile were more likely to be transferred and to have a higher base deficit compared with infants with a cumulative exposure <50th percentile. They also had a lower 10 minute apgar score and required a longer time to spontaneous respiration relative to infants with no hypocarbia.
Table 1.
Maternal and Neonatal Characteristics
| Cumulative Hypocarbia >50th Percentile n=90 | Cumulative Hypocarbia ≤ 50th Percentile n=91 | No Hypocarbia Exposure n=23 | p-values | ||
|---|---|---|---|---|---|
| Maternal Age (years) | Mean ± SD | 27.1 ± 5.8 | 27.3 ± 6.5 | 29.0 ± 5.4 | ns |
| Married | n (%) | 55 (61.1) | 48 (52.7) | 12 (52.2) | ns |
| Age at Randomization (hours) | Mean ± SD | 4.5 ± 1.1 | 4.1 ± 1.3 | 4.3 ± 1.5 | ns |
| Transferred from Birth Hosp. | n (%) | 53 (58.9) | 27 (29.7) | 10 (43.5) | a |
| Male Sex | n (%) | 48 (53.3) | 52 (57.1) | 15 (65.2) | ns |
| Apgar Score<=5 at 5 Min | n (%) | 84 (94.4) | 82 (90.1) | 19 (82.6) | ns |
| Apgar Score <=5 at 10 Min | n (%) | 70 (84.3) | 70 (81.4) | 11 (61.1) | b |
| Gestational Age (wks) | Mean ± SD | 38.9 ± 1.6 | 38.9 ± 1.6 | 38.5 (1.7) | ns |
| Birth Weight (g) | Mean ± SD | 3236 ± 511 | 3452 ± 673 | 3579 ± 757 | a, b |
| Intubation in Delivery Room | n (%) | 85 (94.4) | 87 (95.6) | 19 (82.6) | ns |
| Continued Resusc.at 10 Min. | n (%) | 87 (96.7) | 84 (92.3) | 20 (87.0) | ns |
| Ventilation (days) | Mean ± SD | 6.1 ± 5.5 | 4.9 ± 5.0 | 6.2 ± 7.3 | ns |
| Time to Spont.Resp.>10 Min. | n (%) | 70 (79.5) | 58 (68.2) | 11 (50.0) | b |
| Cord Blood – pH | Mean ± SD | 6.8 ± 0.2 | 6.9 ± 0.2 | 6.9 ± 0.2 | a, b |
| Cord Blood - Base Deficit (mol/l) | Mean ± SD | 21.7 ± 7.0 | 17.1 ± 7.3 | 16.8 ± 10.2 | a |
| Seizures | n (%) | 53 (58.9) | 33 (36.3) | 6 (26.1) | a, b |
| Emergency C-Section | n (%) | 71 (79.9) | 61 (67.0) | 16 (69.6) | ns |
| Moderate Encephalopathy | n (%) | 56 (62.2) | 60 (66.7) | 15 (65.2) | ns |
| Severe Encephalopathy | n (%) | 34 (37.8) | 30 (33.3) | 8 (34.8) | - |
p-value: >50th Pctl Cumulative Exposure to Hypocarbia & <=50th Pctl Hypocarbia <0.05
p-value: >50th Pctl Cumulative Exposure to Hypocarbia & No Hypocarbia <0.05
The respiratory support of the study participants is outlined below: at randomization, 18 infants were treated with high frequency oscillatory ventilation (HFOV), 165 infants were treated with intermittent mandatory ventilation (IMV), five with CPAP and eight with supplemental oxygen by hood or cannula. By 24 hours of age, the ventilatory support was reduced in 50 infants: 18 infants with HFOV, 115 infants with IMV, eight with CPAP, 22 with supplemental oxygen and 34 with no assistance. Ventilator settings at randomization included: an FiO2 of 0.70 ± 0.31 (mean ± SD), rate of 36 ± 15 (mean ± SD), PIP of 22 ± 6 (mean ± SD) and MAP of 9 ± 4 cm H20 (mean ± SD). Among infants on IMV at randomization, infants with severe hypocarbia (minimum PCO2<25mmHg) had higher ventilator settings than those without: rate of 40±16 vs. 34±14 (P=0.03) and product of rate × (PIP-PEEP) of 744±458 vs. 585±345 (P=0.06).
The median PCO2 values throughout the first 12 hours of study intervention for infants with moderate hypocarbia (any PCO2 <35 mmHg) were 26 mmHg pre-randomization, 27 mmHg at randomization, 27 mmHg at 4h, 28 mmHg at 8h and 29 mmHg at 12 hours. The duration of time spent with a PCO2 <35 mmHg, was 8.3 ± 5.7 hours (mean ± SD, range 0-21.3hrs); whereas the duration of time spent with a PCO2 <25 mmHg was 2.5 ± 3.7 hours (mean ± SD, range 0-18.3h). The mean exposure to cumulative hypocarbia (<35mmHg) ± SD of each of the quartiles is shown in Table II. Hypercarbia (PCO2 >50mmHg) was less frequent: 28% of infants had hypercarbia on the first postnatal pre-randomization blood gas and 5.8% to 7.4% of infants had hypercarbia on subsequent blood gases.
Table 2.
Measures of Cumulative Exposure to Hypocarbia
| Quartiles of Cumulative Exposure to PaCO2<35mmHg | Cumulative Hypocarbia Range (mmHg.hrs) | Cumulative Hypocarbia Mean ± SD (mmHg.hrs) | Hours of Hypocarbia Mean ± SD (hrs) | |
|---|---|---|---|---|
| > 75th quartile | 45 | ≥ 116 | 165.3 ± 39.0 | 14.7 ± 3.4 |
| 50-75th quartile | 45 | 59 - 115 | 88.7 ± 17.2 | 10.8 ± 3.7 |
| 25-50th quartile | 45 | 29 - 58 | 42.9 ± 8.2 | 8.3 ± 3.1 |
| ≤ 25th quartile | 46 | 1 - 28 | 12.2 ± 8.1 | 3.5 ± 2.4 |
| No Exposure | 23 | - | - | - |
Death or moderate/severe disability at 18-22 months occurred in 108 of the 204 infants with outcome data: there were 62 deaths (24 in the hypothermia group and 38 in the control group), 43 severely disabled (18 hypothermia and 25 control) and 3 moderately disabled (2 hypothermia and 1 control). Infants with poor outcome had significantly lower minimum PCO2 concentrations (median 22 vs. 26 mmHg) and greater fluctuations in PCO2 concentrations (difference in maximum and minimum PCO2, SD of PCO2) (Table III). Multiple logistic regression analysis performed to estimate the relationship between minimum, maximum PCO2 and fluctuations in PCO2 and death/disability revealed that only minimum PCO2 was a significant predictor of outcome, odds ratio 2.0 (95% CI 1.1-3.4) (P=0.015); this model fit the data well (AUC=0.856). Cumulative exposure to moderate hypocarbia (PCO2 <35mmHg) also was associated with the combined outcome of death or disability (Table III). Death or disability increased with greater cumulative hypocarbia (35% among neonates with no exposure to 64% in the highest exposure group) (Figure). Adding birth weight to the model did not change the significance of the results (data not shown). Time to spontaneous respiration > 10 minutes was significantly associated with death or disability (P<0.001), as was the number of ventilator days (P=0.009), pH (P=0.005), and cumulative hypocarbia (P=0.049). The “presence of clinical seizures” was not significantly associated with death or disability. Moreover, the relationship between hypocarbia and death/disability was not affected when “presence of clinical seizures” was included in the multiple regression model. Cumulative exposure to hypercarbia also was evaluated; hypercarbia was not a significant predictor of death or disability (data not shown).
Table 3.
Predictors of Poor Outcome [Death/Disability]
| Median (25th & 75th Percentile) PCO2 Values (mmHg) | Adjusted Analyses* | ||||
|---|---|---|---|---|---|
| Measures of PCO2 Exposure | Death or Disability | No Death Disability | p-value** | OR & 95% CI*** | p-value |
| Minimum PCO2 to 12h | 22 (20 - 29) | 26 (21 - 32) | 0.0202 | 2.0 (1.1 - 3.4) | 0.0151 |
| Maximum PCO2 to 12h | 48 (38 - 66) | 42 (37 - 59) | 0.1768 | 1.1 (1.0 - 1.3) | 0.1423 |
| Difference in Max. & Min. PCO2 | 23.5 (13 - 42) | 18 (9 - 32) | 0.0247 | 1.1 (0.9 - 1.2) | 0.3986 |
| Standard Deviation in PCO2 Measurements | 10.3 (5.7-16.9) | 7.8 (4.3-13.0) | 0.0435 | 1.2 (0.9 - 1.6) | 0.3169 |
| Logistic Regression Model with Cumulative Exposure | Unadjusted Odds Ratios | Adjusted Odds Ratios | ||
| Cumulative Exposure to Hypocarbia (<35mmHg) | OR & 95%CI | p-value | OR & 95%CI | p-value |
| > 75th quartile (>=116 mmHg.hrs) | 4.1 (1.4 - 12.5) | 0.0536 | 6.9 (1.5 - 31.9) | 0.0490 |
| 50-75th quartile (59-115 mmHg.hrs) | 3.4 (1.2 - 10.1) | - | 7.7 (1.8 - 33.4) | - |
| 25-50th quartile (29-58 mmHg.hrs) | 2.6 (0.9 - 7.8) | - | 4.4 (1.0 - 19.4) | - |
| < 25th quartile (1-28 mmHg.hrs) | 1.6 (0.6 - 4.8) | - | 2.5 (0.6 - 10.4) | - |
| Initial Level of HIE – Severe vs. Moderate | 6.0 (3.0 - 12.0) | <0.0001 | 3.4 (1.5 - 7.7) | 0.0043 |
| Study Treatment Group – Control vs Hypothermia | 2.2 (1.2 - 3.9) | 0.0080 | 3.3 (1.5 - 7.0) | 0.0024 |
| Time to Spontaneous Respiration >10min vs < 10min | 8.1 (3.8 - 17.1) | <0.0001 | 5.0 (2.1 - 11.7) | 0.0002 |
| pH at Randomization (0.1 point decrease) | 1.4 (1.2 - 1.7) | 0.0009 | 1.5 (1.1 - 1.9) | 0.0047 |
| Days on Ventilation (1 day increase) | 1.2 (1.0 - 1.2) | 0.0009 | 1.1 (1.0 - 1.2) | 0.0088 |
| AUC c-statistic | 0.861 | |||
Adjusted for initial level of encephalopathy, study treatment group, time to spontaneous respiration >10 minutes, systemic pH at randomization & days on ventilation
Wilcoxon
Odds Ratios are increased odds of death/disability associated with a 10 mmHg decline in the PCO2 measure.
Figure 1.
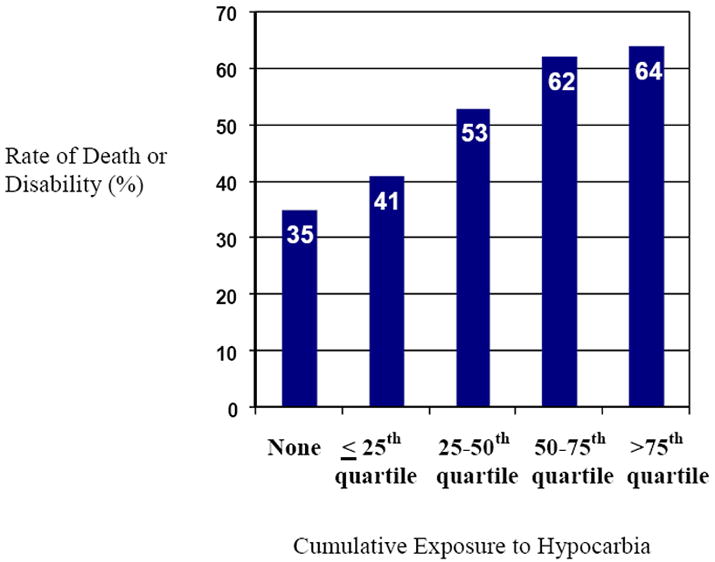
Rate of Primary Outcome with Increasing Cumulative Exposure to Hypocarbia (PCO2 < 35mmHg)
The relationship between hypocarbia and adverse outcome was examined for the hypothermia group of participants and the intensive care alone group separately. There was no significant association between hypocarbia and poor outcome. Though the odds of higher death/disability tended to increase with increasing exposure to hypocarbia, the results were statistically not significant for both the hypothermia (P=0.18) and the intensive care alone groups (P=0.42). The minimum PCO2 among infants treated with hypothermia was 24.3 mmHg compared with 26.2 mmHg in infants treated with intensive care alone (P=0.004). Interestingly, minimum PCO2 was associated with poor outcome in infants treated with intensive care alone [OR 2.19 (95% CI 1.04-4.63) (P=0.04)], but not among infants treated with hypothermia [OR 1.64 (95% CI 0.71-3.78) (P=0.25)].
In addition, we examined the relationship between hyperoxia and hypocarbia, and between hyperoxia and death or disability for a subset of infants with arterial blood gases (n=150). This sub-group analysis is limited by the fact that the source of the blood gas was available only for the first postnatal gas. Several measures of severe and moderate hyperoxia were assessed (PaO2 >200mmHg and >100 mmHg respectively): any hyperoxia, cumulative hyperoxia, number of hours of hyperoxia and cumulative hours of hyperoxia. We found low correlation between the hyperoxia measures and cumulative hypocarbia (of at most, rho= -0.366 for cumulative hours of PaO2 >200mmHg and rho= -0.383 for cumulative hours of PaO2 >100mmHg). No hyperoxia measure was associated with outcome significantly; thus hyperoxia was not used as a covariate in the multivariate analysis.
DISCUSSION
We report an association between hypocarbia soon after birth in neonates with hypoxic ischemic encephalopathy and poor outcome. Low PCO2 concentrations within the first 16 hours of life were associated with an increased risk of death or disability directly related to the degree and severity of hypocarbia. Both minimum PCO2 and cumulative PCO2 <35mmHg were associated with poor outcome at 18-22 months of age. Whether hypocarbia is an early marker or a risk factor for poor neurodevelopmental outcome remains to be determined. Even though non-ventilated infants or infants on minimal ventilatory support can develop hypocarbia, they rarely have PCO2 concentrations <25 mmHg22. Indeed, other studies have reported a much lower incidence and severity of hypocarbia among neonates with HIE22,23. These reports focus on infants with milder encephalopathy and milder respiratory depression (less mechanical ventilation). The majority of infants in our study received significant ventilatory assistance and resuscitation at birth, so early management may have contributed to hypocarbia. Other mediating factors may have contributed as well. Low PCO2 concentrations may have been produced by the combined effects of the intervention, the severity of brain injury (decreased CO2 production), and the infant’s own respiratory drive and ability to correct metabolic acidosis. An important consideration is whether avoidance of hypocarbia is possible in the first 16 hours of life (coincident with the putative reperfusion period in many animal models of HIE) and whether this will lead to improved outcomes. In other words, is hypocarbia a modifiable risk factor or simply a marker of poor outcome?
Klinger et al reported that hyperoxia in addition to hypocarbia may be detrimental following HIE12. In a retrospective cohort study of 218 infants born between 1985 and 1995 treated at the Hospital for Sick Children Toronto, hypocarbia and hyperoxia within two hours of life were associated with an increased risk of death or disability, with risk of death/disability being highest among infants with both hypocarbia and hyperoxia. Our study found no association between hyperoxia and death or disability; however time to spontaneous respiration (often closely linked to oxygen exposure) was strongly associated with poor outcome.
The blood gases in our study were temperature-corrected for the hypothermia group which may have influenced our results. Temperature correction of blood gases for mild therapeutic hypothermia in neonatal HIE is controversial13. Recent studies comparing alpha-stat management (no correction) with pH-stat management (PCO2 corrected to the patient’s actual body temperature) in animal models of deep hypothermia report a significant increase in tissue oxygenation and cortical blood flow and a decrease in infarct volume and cerebral edema in studies using temperature correction24,25. However, results are inconsistent in human studies26,27,28; and the effects of alpha-stat vs. pH stat management may vary with the degree of hypothermia (mild to moderate hypothermia vs. deep hypothermia) and the timing of management (e.g., early reperfusion vs. later management)29. The partial pressure of PCO2 in blood decreases with decreasing temperatures; so had alpha-stat management been employed in our study, a greater degree of ventilator-dependent hyperventilation may have been observed in the hypothermia group (in an attempt to maintain PCO2 concentration in the apparently normal range). Moreover, PaO2 concentrations would have appeared considerably higher than values during normothermia, potentially impacting oxygen use. Data favoring temperature correction vs. no correction in neonatal HIE are limited15; further investigation is needed.
This study raises important issues regarding the complexity of the early postnatal ventilatory management of neonates with HIE. There is biological plausibility for hypocarbia to exacerbate brain injury: low carbon dioxide tension may impact cerebral perfusion, restoration of cellular energy metabolism, oxygen transport, oxygen extraction and removal of potentially neurotoxic metabolites 1,4,5,30. Important questions to consider include whether alpha-stat or pH-stat management is better for neonatal hypothermia for HIE. Second, should cooled infants be started on lower initial ventilator settings or weaned more rapidly in view of their lower metabolic rate (and consequent lower CO2 production)? Third, will avoidance of hypocarbia improve outcome; and lastly, will hypercarbia and oxygenation impact outcome?
Neither maximum pCO2 nor cumulative exposure to hypercarbia (the difference between the sampled PCO2 and 50 mmHg multiplied by the duration of time spent above 50 mmHg summed from birth-12h of intervention) were associated with outcome in our study. However, these results should be interpreted with caution as few infants had hypercarbia during this brief and very early time interval. Hypercarbia may have ensued later in the course of HIE or following extubation, and this was not examined in our study. Hypercarbia has been associated with altered risk of neurological outcome18-19.
There are some additional limitations to our data. Our study is underpowered to answer the question of whether there is an interaction between hypocarbia and outcome in neonates treated with therapeutic hypothermia versus those treated with intensive care alone. Moreover, we evaluated a very brief period of time (study randomization to 12 hours of intervention) and utilized intermittent blood gas sampling from a combination of arterial, venous and capillary blood samples. It is clearly simplistic to assume that a single blood gas sample can accurately represent a four hour period of ventilator delivered breaths or that only the first 12 hours of intervention are important. Moreover, infants with significant brain injury may have patterns of both spontaneous hyperventilation and hypoventilation which may lead to significant intermittent fluctuations in pCO2 between blood gas measurements. Continuous sampling of pCO2 may be a better measurement of this phenomenon, but such sampling is technically difficult for cooled infants. Current transcutaneous monitoring techniques are not feasible with whole body cooling, and continuous end tidal CO2 monitoring lacks adequate precision.
The PCO2 values in our study were obtained from a combination of arterial, venous and capillary blood samples and may have underestimated the overall degree of hypocarbia. In addition, the severity of hypocarbia may have been affected by our clinical strategy to temperature correct the blood gases for the hypothermia group alone; the ventilatory management of cooled infants targeted normal values for temperature corrected blood gas samples. Arterial samples from all participants and a consistent strategy of temperature correction of blood gases for all participants would have been preferable. Arterial sampling would have provided important information on oxygenation as well. The assumption that infants with an initial arterial blood gas had subsequent gases drawn arterially may not have been accurate. Finally, insight into the mechanism of injury is needed and may be addressed in part with the aid of adjunctive cerebral monitoring, cerebral blood flow studies and neuroimaging. These considerations may guide future prospective studies in neonates with hypoxic ischemic encephalopathy.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Supported by a grant from the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for the NICHD Neonatal Research Network’s Whole Body Cooling for Hypoxic Ischemic Encephalopathy Study. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, A.D. and J.L. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
List of abbreviations
- HIE
Hypoxic-ischemic encephalopathy
- NICU
Neonatal Intensive Care Unit
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
- GMFCS
Gross Motor Function Classification System
- NICHD
National Institute of Child Health and Development
- NRN
Neonatal Research Network
- SD
Standard Deviation
- IQR
Interquartile Range
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati.
Case Western Reserve University Rainbow Babies & Children’s Hospital (GCRC M01 RR80, U10 HD21364) – Avroy A. Fanaroff, MD; Michele C. Walsh, MD MS; Deanne E. Wilson-Costello, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (GCRC M01 RR8084, U10 HD27853) – Edward F. Donovan, MD; Kurt Schibler, MD; Jean J. Steichen, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA.
Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (GCRC M01 RR30, U10 HD40492) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Melody B. Lohmeyer, RN MSN.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (GCRC M01 RR39, U10 HD27851) – Barbara J. Stoll, MD; Lucky Jain, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Rosemary D. Higgins, MD; Elizabeth M. McClure, MEd.
Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (GCRC M01 RR750, U10 HD27856) – James A. Lemons, MD; Brenda B. Poindexter, MD MS; Anna M. Dusick, MD FAAP; Diana D. Appel, RN BSN; Lucy C. Miller, RN BSN CCRC; Leslie Richard, RN.
RTI International (U01 HD36790) – W. Kenneth Poole, PhD; Abhik Das, PhD; Betty K. Hastings; Jamie E. Newman, PhD MPH; Carolyn Petrie Huitema, MS.
Stanford University Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (GCRC M01 RR70, U10 HD27880) – David K. Stevenson, MD; Krisa P. Van Meurs, MD; Susan R. Hintz, MD MS; M. Bethany Ball, BS CCRC.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (GCRC M01 RR32, U10 HD34216) – Waldemar A. Carlo, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; Maynard R. Rasmussen, MD; David Kaegi, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT.
University of Miami Holtz Children’s Hospital (GCRC M01 RR16587, U10 HD21397) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN.
University of Rochester Golisano Children’s Hospital at Strong (GCRC M01 RR44, U10 HD40521) – Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Gary J. Myers, MD; Linda J. Reubens, RN CCRC; Diane Hust, MS RN CS.
University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas (GCRC M01 RR633, U10 HD40689) – Abbot R. Laptook, MD; Pablo J. Sánchez, MD; R. Sue Broyles, MD; Roy J. Heyne, MD; Susie Madison, RN; Jackie F. Hickman, RN; Gaynelle Hensley, RN; Nancy A. Miller, RN.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Brenda H. Morris, MD; Pamela J. Bradt, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia I. Franco, RNC MSN; Anna E. Lis, RN BSN; Georgia E. McDavid, RN.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Yvette R. Johnson, MD MPH; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN; Deborah Kennedy, RN BSN.
Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Abbot R. Laptook, MD; Betty R. Vohr, MD; Angelita M. Hensman, RN BSN; Lucy Noel.
Yale University Yale-New Haven Children’s Hospital (CCTS UL1 RR24139, GCRC M01 RR6022, U10 HD27871) – Richard A. Ehrenkranz, MD; Patricia Gettner, RN; Elaine Romano, MSN.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson DF, Pastuszko A, DiGiacomo JE, Pawlowski M, Schneiderman R, Delivoria-Papadopoulos M. Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J Appl Physiology. 1991;70:2691–2696. doi: 10.1152/jappl.1991.70.6.2691. [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Willumsen L, Hauerberg J, Pederson DB, Juhler M. Effects of graded hyperventilation on cerebral blood flow autoregulation in experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2000;20:718–25. doi: 10.1097/00004647-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 4.Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM. Effect of carbon dioxide on background electrical activity and fractional oxygen extraction in very low birth weight infants just after birth. Pediatr Res. 2005;58:579–585. doi: 10.1203/01.pdr.0000169402.13435.09. [DOI] [PubMed] [Google Scholar]
- 5.Fritz KI, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of moderate hypocapnic ventilation on nuclear DNA fragmentation and energy metabolism in the cerebral cortex of newborn piglets. Pediatr Res. 2001;50:586–589. doi: 10.1203/00006450-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Fritz KI, Zubrow AB, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. The effect of hypocapnia (PCO2 27mmHg) on CaM kinase IV activity, Bax/Bcl-2 protein expression and DNA fragmentation in the cerebral cortex of newborn piglets. Neuroscience Letters. 2003:211–215. doi: 10.1016/j.neulet.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 7.Fritz KI, Zubrow AB, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. The effect of moderate hypocapnic ventilation on nuclear Ca-ATPase activity, nuclear Ca flux, and Ca/Calmodulin Kinase IV activity in the cerebral cortex of newborn piglets. Neurochem Res. 2004;29:791–796. doi: 10.1023/b:nere.0000018852.85899.85. [DOI] [PubMed] [Google Scholar]
- 8.Pirot AL, Fritz KI, Ashraf QA, Mishra OP, Delivoria-Papadopoulos M. Effects of severe hypocapnia on expression of Bax and Bcl-2 proteins, DNA fragmentation, and membrane peroxidation products in cerebral cortical mitochondria of newborn piglets. Neonatology. 2007;91:20–27. doi: 10.1159/000096967. [DOI] [PubMed] [Google Scholar]
- 9.Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, Spitzer AR. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high frequency jet ventilation. Pediatrics. 1996;98:918–924. [PubMed] [Google Scholar]
- 10.Shankaran S, Langer JC, Kazzi SN, Laptook AR, Walsh M. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia (PVL) in low birth weight (LBW) infants. Pediatrics. 2006;118:1654–1659. doi: 10.1542/peds.2005-2463. [DOI] [PubMed] [Google Scholar]
- 11.Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilation-related risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50:712–719. doi: 10.1203/00006450-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Klinger G, Beyene J, Shah P, Perlman M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch Dis Child Fetal Neonatal Ed. 2005;90:49–52. doi: 10.1136/adc.2003.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groenendaal F, De Vooght KMK, van Bel F. Blood gas values during hypothermia in asphyxiated term neonates. Pediatrics. 2009;123:2008–1955. doi: 10.1542/peds.2008-1955. [DOI] [PubMed] [Google Scholar]
- 14.Laptook AR, Corbett RJ, Sterett R, Garcia D, Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res. 1995;38:919–925. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041–51. doi: 10.1097/01.CCM.0000069731.18472.61. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole Body Hypothermia for Neonates with Hypoxic-Ischemic Encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 17.Fabres J, Carlo WA, Philips V, Howard G, Ambalavanan N. Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics. 2007;119:299–305. doi: 10.1542/peds.2006-2434. [DOI] [PubMed] [Google Scholar]
- 18.Fritz KI, Zubrow A, Mishra OP, Delivoria-Papadopoulos M. Hypercapnia-induced modifications of neuronal function in the cerebral cortex of newborn piglets. Pediatr Res. 2005;57:299–304. doi: 10.1203/01.PDR.0000148718.47137.9B. [DOI] [PubMed] [Google Scholar]
- 19.Vannucci RC, Towfighi J, Brucklacher RM, Vannucci SJ. Effect of extreme hypercapnia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res. 2001;49:799–803. doi: 10.1203/00006450-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev MedChild Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant Development II. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 22.Nadeem M, Murray D, Boylan G, Dempsey EM, Ryan CA. Blood carbon dioxide levels and adverse outcome in neonatal hypoxic-ischemic encephalopathy. Amer J Perinatology. 2010;27:361–365. doi: 10.1055/s-0029-1243309. [DOI] [PubMed] [Google Scholar]
- 23.Engle WD, Laptook AR, Perlman JM. Acute changes in carbon dioxide tension and acid-base status and early neurologic characteristics in term infants following perinatal asphyxia. Resuscitation. 1999;42:11–17. doi: 10.1016/s0300-9572(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 24.Kollmar R, Frietsch T, Georgiadis D, Schabitz W, Waschke KF, Kuschinsky W, Schwab S. Anesthesiology. 2002;97:868–874. doi: 10.1097/00000542-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Duebener LF, Hagino I, Sakamoto T, Mime LB, Stamm C, Zurakowski D, Schäfers HJ, Jonas RA. Effects of pH management during deep hypothermic bypass on cerebral microcirculation: alpha-stat versus pH-stat. Circulation. 2002;106:I103–I108. [PubMed] [Google Scholar]
- 26.Patel RL, Turtle MR, Chambers DJ, James DN, Newman S, Venn GE. Alpha-stat acid base regulation during cardiopulmonary bypass improves neuropsychologic outcome in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:1267–1279. doi: 10.1016/s0022-5223(96)70230-1. [DOI] [PubMed] [Google Scholar]
- 27.du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1001. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 28.Bellinger DC, Wypij D, du Plessis AJ, Rappaport LA, Riviello J, Jonas RA, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Yin X, Ye J. Effects of pH management during deep hypothermic bypass on cerebral oxygenation: alpha-stat versus pH-stat. J Zhejiang Univ SCI. 2004;5:1290–1297. doi: 10.1631/jzus.2004.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brun NC, Griegen G. Cerebrovascular responses to carbon dioxide as detected by near-infrared spectrophotometry: comparison of three different measures. Pediatr Res. 1994;36:1–4. doi: 10.1203/00006450-199407001-00004. [DOI] [PubMed] [Google Scholar]


