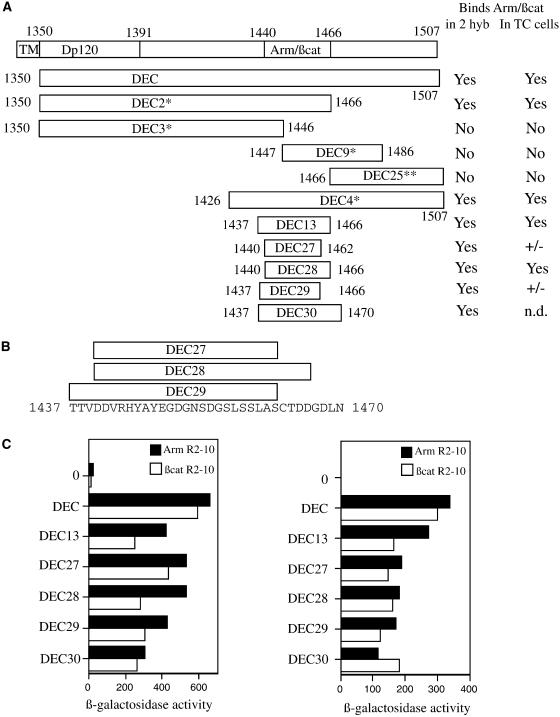
Figure 1.
Mapping the minimal binding site on DEC cytoplasmic tail for β-catenin (βcat) and Arm using the yeast two-hybrid (2 hyb) system. (A) Schematic representation of the DEC derivatives used in our analyses, with ability to bind Arm/β-catenin in either yeast or mammalian cells summarized in the right-hand columns. TM, transmembrane; TC, tissue culture. *Data from Pai et al. (1996). (B) Sequence of the minimal binding region of DE-cadherin, with the boundaries of the smallest DEC derivatives indicated. (C) All of the DEC derivatives bind to both fragments of Arm and β-catenin in yeast. The full-length DE-cadherin cytoplasmic domain (DEC), or smaller derivatives of DEC (diagrammed in A and B), fused to the Gal4 transcriptional activation domain, were transformed into yeast cells along with portions of Arm or β-catenin fused to the LexA DNA-binding domain. Average β-galactosidase values are shown for each DEC derivative together with the full Arm repeat region of Arm or β-catenin (Arm R1–12 or βcat R1–12, left), or a smaller fragment of the Arm repeat region (Arm R2–10 or βcat R2–10, right). 0, background level of β-galactosidase activity with no DEC fragment fused to Gal4. **DEC 25 was tested against only Arm R1–12. Its β-galactosidase value was 14.4 U, compared with 18.3 U for the negative control.