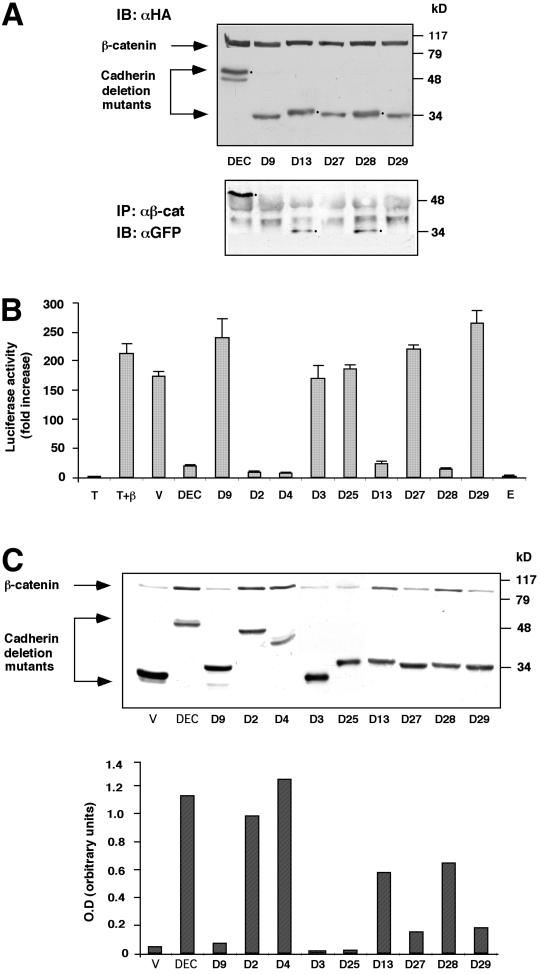
Figure 2.
Analysis of the ability of different fragments of the DEC cytoplasmic tail to interact with β-catenin, affect its stability, and inhibit β-catenin–mediated transactivation. (A) The ability of selected GFP-DEC derivatives to coimmunoprecipitate with cotransfected HA-tagged β-catenin was determined by immunoprecipitation (IP) from 293T cells transfected with HA-tagged β-catenin and GFP-tagged DEC constructs with anti-GFP antibody, followed by Western blotting with anti-HA antibody. The total level of transfected β-catenin and DEC constructs was determined by immunoblotting (IB) with anti-HA-antibody. (B) 293T cells were transfected with GFP-tagged derivatives of the DEC cytoplasmic tail (DEC) or the full-length mammalian E-cadherin tail (E), along with β-catenin (β), a LEF/TCF reporter plasmid (T), and Lac Z. Luciferase activity was determined from duplicate plates as fold activation after normalizing for transfection efficiency by measuring β-galactosidase activity. T, cells were transfected with the reporter plasmid alone; V, cells transfected with the reporter plasmid, HA-tagged β-catenin and the GFP-vector used for the construction of the cadherin derivatives. (C) The cadherin derivatives used in B were transfected into CHO cells, and their ability to protect the endogenous β-catenin from degradation was determined by analyzing the level of β-catenin expressed in the DEC mutant-transfected cells by Western blotting with anti-β-catenin antibody. The level of expression of DEC constructs was determined by immunoblotting with an antibody against the GFP tag. Quantitation of the β-catenin level expressed in CHO cells was carried out by normalizing the intensity of the β-catenin bands shown to those of the DEC band for each derivative.