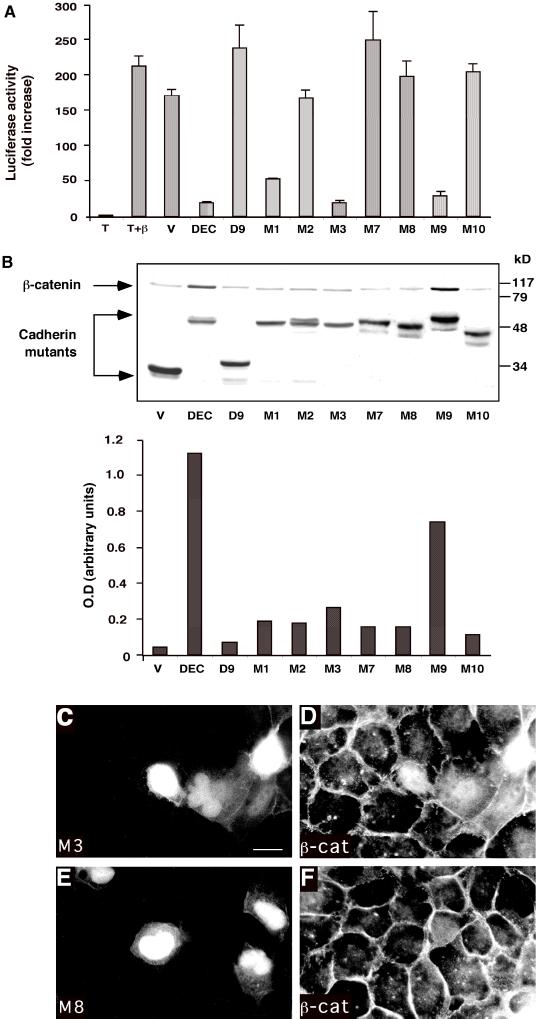
Figure 6.
Analysis of the effect of clustered point mutations in the minimal Arm-binding domain of DEC on its capacity to interact with β-catenin, protect it from degradation, inhibit β-catenin/LEF-mediated transactivation, and affect β-catenin organization. (A) The ability of clustered point mutations (diagrammed in Figure 5A) to affect β-catenin/LEF-1–mediated transactivation in 293T cells was examined as described in Figure 2B. (B) The ability to protect β-catenin from degradation was examined in CHO cells, and the levels of β-catenin were quantified as described in Figure 2C. Because the samples were originally analyzed on the same gel with the samples shown in Figure 2C, the control samples (V, DEC, and D9) are shown again. (C–F) MDCK cells were transfected with GFP-tagged DECM3 (C, M3) and DECM8 (E, M8), and the organization of the endogenous β-catenin (β-cat) in the respective samples (D and F) was determined by double fluorescence microscopy. Bar, 10 μm.