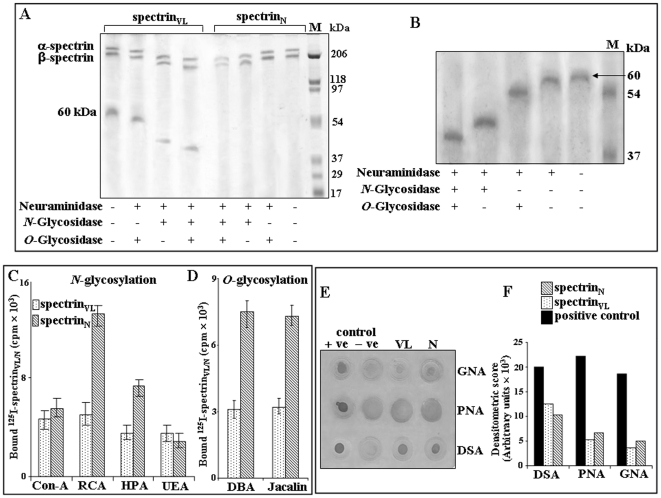
Figure 4. Demonstration of N-and O-glycosylation.
A. Demonstration of N-and O-glycosylation of spectrin by enzyme deglycosylation. Equal amount (5 µg) of purified spectrinVL and spectrinN was treated with neuraminidase from Arthrobacter ureafaciens to remove the terminal sialic acids and subsequently desialylated spectrinVL and spectrinN was incubated separately with N-glycosidase F, O-glycosidase or a combination of N- and O-glycosidase as indicated. SpectrinVL/N before and after the respective enzyme treatments were analyzed by SDS-PAGE as described in Materials and Methods. B. Demonstration of sialylation, N- and O-glycosylation in 60 kDa fragment. Gel-eluted purified 60 kDa fragment (1.0 µg) was initially desialylated with Arthrobacter ureafaciens neuraminidase overnight at 37°C. Subsequently the desialylated 60 kDa fragment was treated separately with N-glycosidase F, O-glycosidase F or a combination of both and analyzed by SDS-PAGE (7.5%) along with the untreated protein as described in Materials and Methods. Gel was stained with silver staining method. Lane M shows molecular weight standards. C–D. Demonstration of N- and O-glycosylation by lectin binding with 125I-spectrinVL/N. Fixed concentrations of 125I-spectrinVL/N were processed separately to demonstrate their binding with several Sepharose/agarose bound ConA, RCA, HPA, UEA, DBA and Jacalin lectins (25 µl bead volume) having different sugar-linkage specificity as described in Materials and Methods. E–F. Demonstration of N- and O-glycosylation by lectin binding with DIG-glycan. E. Equal amount (2.0 µg) of spectrinVL and spectrinN was dot blotted on NC-paper and analyzed by DIG-glycan and differentiation kit using several lectins (GNA, PNA, DSA) following manufacturer's protocol. F. Representative bar graph of densitometric scores of corresponding spots.