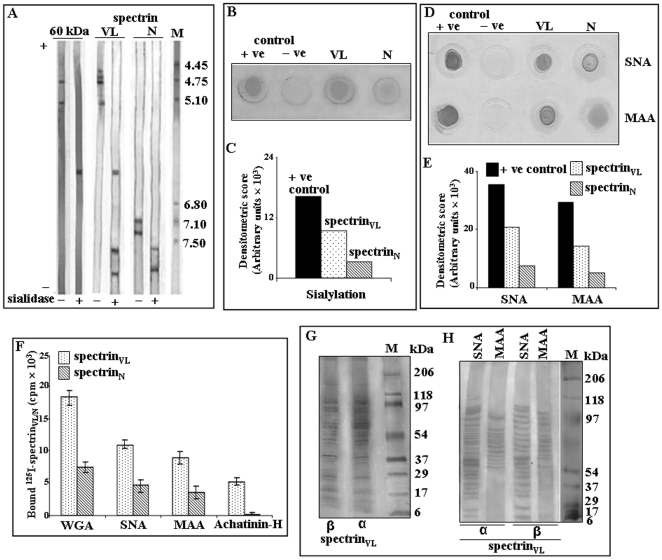
Figure 5. Presence of Neu5Ac and Neu5,9Ac2 in spectrinVL by biochemical methods.
A. Enhanced sialylation demonstrated by IEF. Equal amounts (3.0 µg) of purified spectrinVL, 60 kDa band and spectrinN before and after removal of sialic acids were analyzed by IEF within a pH gradient of 3–10 and the respective bands visualized by silver staining. Lane M shows the pI markers. B–C. Enhanced sialylation in spectrinVL. Equal amount (1.0 µg) of purified spectrinVL and spectrinN was analyzed by using DIG-glycan detection kits and total sialylation was compared based on the densitometric scores of spots (B). Representative bar graph of densitometric scores of corresponding spots (C). D–E. Detection of linkage-specific terminal sialic acids in spectrinVL. Equal amount (2.0 µg) of spectrinVL and spectrinN was dot blotted on NC-paper and analyzed by DIG glycan and differentiation kit using SNA and MAA lectins following manufacturer's protocol (D). Densitometric scores of corresponding spots are shown as bar graph (E). F. Binding of 125I-spectrinVL/N with several sialic acid binding lectins. To demonstrate the presence or absence of terminal sialic acids, a fixed concentrations of 125I-spectrinVL/N were analyzed by binding with Sepharose/agarose bound WGA, SNA, MAA, Achatinin-H (25 µl bead volume) having specificity towards linkage specific sialic acids as described in materials and methods. Bound radioactivity of 125I-spectrinVL/N was measured by Gamma-counter and represented as bar graphs. G–H. Detection of sialylated tryptic fragments in spectrinVL. The α and β subunits of purified spectrinVL were digested separately by restricted amount of trypsin. Such controlled digested and extracted tryptic fragments were dried and redissolved and an aliquot was separated in SDS-PAGE (7.5%–15% gradient) (G). Subsequently the presence of sialic acids on resulting tryptic fragments was analyzed by binding with SNA-agarose and MAA-agarose separately and followed by electrophoresis on SDS-PAGE (7.5%–15% gradient) (H) as described in Materials and Methods. Lane M shows the molecular weight standerds.