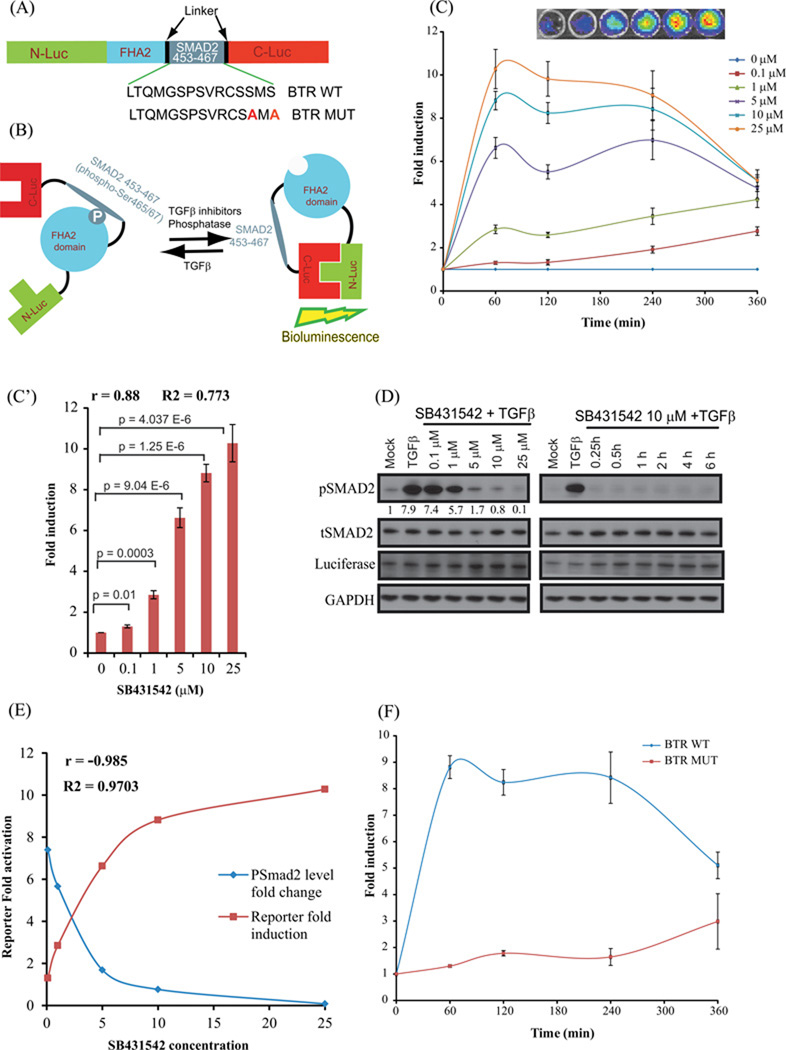
Figure 1. Construction and Validation of bioluminescent TGFβ reporter (BTR) with TGFBR1 kinase inhibitor, SB431542.
(A) The domain structure of the BTR construct: Two versions of the BTR construct were developed; the BTR-WT construct contains the wild-type Smad2 C-terminus, aa 463–467, and the BTR-MUT construct in which alanines were substituted for both serines of the SXS motif. (B) The mechanism of action of the BTR reporter involves TGFBR1 kinase dependent phosphorylation of the Smad2 target peptide which results in its interaction with the FHA2 domain. In this form, the reporter has minimal bioluminescence activity. In the absence of the kinase activity, the target peptide no longer interacts with the FHA2 domain allowing the N-Luc and C-Luc domains to re-associate, restoring bioluminescence activity. (C) A549 cells stably expressing the BTR-WT reporter were treated with increasing concentrations of SB431542 (0.1, 1, 5, 10, and 25µM) in presence of TGFβ (10ng/mL) and bioluminescence was measured serially from baseline through 6 h. Insert displays a representative bioluminescent image of BTR reporter response with increasing concentrations of SB431542 treated cells. The change in bioluminescence activity over mock treatment (DMSO) levels was calculated and plotted as fold induction. Bioluminescent measurements are in triplicates, error bars denote SEM. (C’) Reporter fold induction with SB431542 concentration is plotted to illustrate dose-dependent increase in the reporter activity. Pearson correlation coefficient (r), goodness of fit (R2) and statistical significance (p) is calculated and shown on the plot. (D) Cell lysates were prepared and analyzed by Western blot using anti-phospho-(S465–S467) Smad2, total Smad2, luciferase, and GAPDH antibodies. Level of pSmad2 was measured using imageJ. (E) Pearson correlation coefficient was calculated between pSmad2 level change and reporter fold induction. A very strong negative correlation (r=−0.985) was observed. (F) A549 cells stably expressing BTR-WT and BTR-MUT reporters were treated in triplicate with 10 µM SB431542 and bioluminescence was measured serially for 6 h. The change in bioluminescence activity over vehicle control was calculated and plotted as fold change.