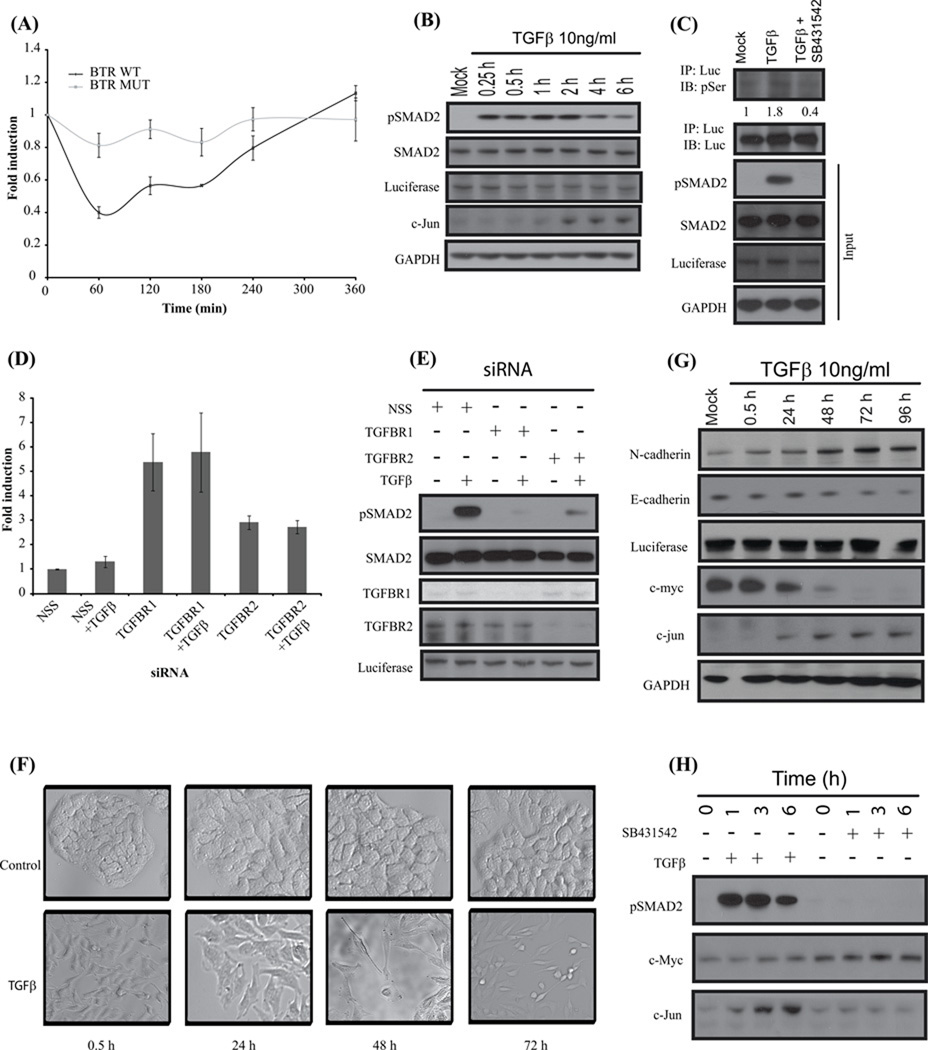
Figure 2. BTR reporter activity is dependent on reporter substrate phosphorylation through TGFβ signaling.
(A) A549 cells stably expressing BTR-WT reporters and BTR-MUT treated with TGFβ (10ng/ml) and bioluminescence measured serially for 6 h. (B) Cell lysates prepared and analyzed by Western blot against anti-phospho-(S465–S467) Smad2, total Smad2, luciferase, C-jun and GAPDH antibodies. (C) BTR-WT reporter expressing A549 cells were treated with TGFβ (10ng/ml) or TGFβ and SB431542 (10 µM) for 1 h, cell lysates were prepared and immunoprecipitated with luciferase specific antibody. The level of substrate phosphorylation was determined by Western blot analysis with phospho-Ser antibody. The immunoblot was probed with luciferase specific antibody as a control and the input lysate was probed against phospho-(S465–S467) Smad2, total Smad2, luciferase and GAPDH. (D) A549 cells stably expressing the BTR-WT reporter were transfected with 100nM siRNA for 72 h, TGFβ was added for an additional 1 h, and reporter activity was measured. Fold change in reporter activity was calculated from change in non-targeted siRNA (NSS). Error bars denote SEM. (E) Cell lysates were prepared and analyzed by Western blot against anti-phospho-(S465–S467) Smad2, total Smad2, TGFBR1, TGFBR2, and luciferase. (F) A549 BTR-WT cells were serum-starved and treated with TGFβ (10ng/ml) for 72 h demonstrating epithelial-to-mesenchymal transition. (G) Cell lysates of A549-BTR WT cells treated with TGFβ for 0.5, 24, 48, 72, and 96 hours were probed with antibodies against N-cadherin, E-cadherin, luciferase, c-jun, c-myc and GAPDH. (H) Western blots analysis of A549 BTR-WT cells treated with 10µM SB431542 or 10ng/ml TGFβ for 0, 1, 3, 6 h with antibodies against phospho-(S465–S467) Smad2, c-myc and c-jun.