Fig 4.
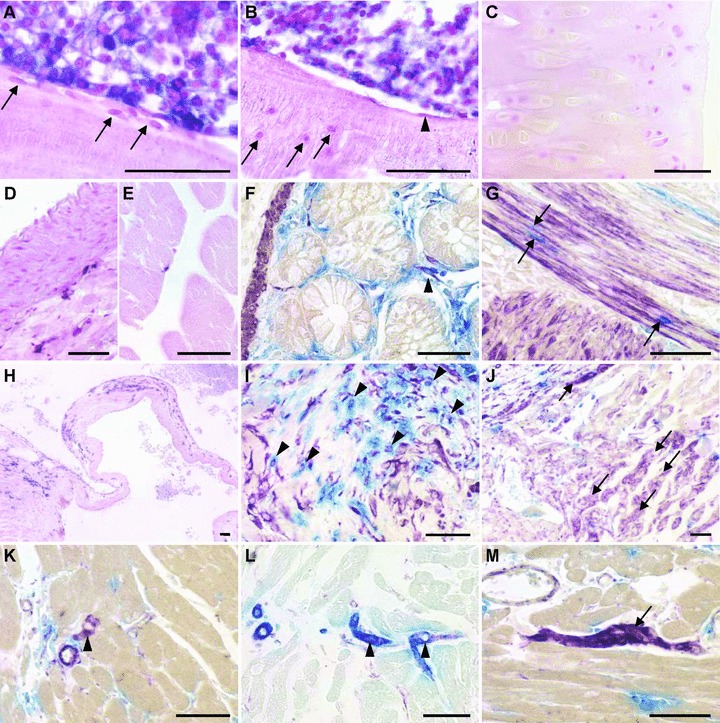
Renewal of mesenchymal cells in irradiated wt F344 rats reconstituted with unfractionated BM from ALPP-tg F344 donors, 4–6 months after BMT. Sections of methylmethacrylate-embedded bones from reconstituted wt F344 rats do not show evidence of ALPP+ BM-derived osteoblasts (A, arrows), osteocytes (B, arrows), bone lining cells (B, arrowhead) or chondrocytes (C). In contrast, BMC were strongly positive for the marker enzyme (A, B). Histochemical detection of the marker enzyme in paraffin sections shows absence of BM-derived smooth muscle cells in aorta (D), and absence of ALPP-expressing muscle fibres in skeletal muscle (E). Cells staining double positive (arrowhead) for ALPP activity (blue) and anti- SMA (purple) were rarely present in the gut (F). In contrast, BM-derived single ALPP+ endothelial-like cells (blue) located between the smooth muscle cells could be frequently found (G, arrows). In the heart, we found numerous ALPP+ cells nearby valvular insertion sites and in the valvular leaflets, here at 4 months after BMT (H). Combination of ALPP-staining (blue) with immunohistochemical staining against vimentin (purple) or SMA (not shown) demonstrated that the BM-derived cells in the valvular insertion areas partially also stained positive for vimentin, suggesting a fibroblast-like nature (I, arrowheads). Numerous single vimentin+ cells with cardiomyocyte-like morphology could also be found at the valvular insertion sites, indicated by arrows (J), as well as rarely in the working myocardium (arrowhead in K), throughout the study period. In rare cases, these vimentin+ (purple) cardiomyocyte-like cells stained also positive for SMA (blue), possibly reflecting early differentiated cardiomyocytes (L). Moreover, in epicardial areas of the heart base, we found several clusters of vimentin+ ALPP– cells (M), potentially representing a cardiac resident stem cell pool. The 5-μm-thick paraffin and methylmethacrylate sections shown in (A–E) and (H) were stained for ALPP enzyme activity with BCIP/NBT (purple) overnight at RT after heat pre-treatment, and were counterstained with nuclear fast red. The paraffin sections in (F–G) and (I–M) were stained for ALPP enzyme activity using Vector Blue, and immunostained against vimentin or SMA using Vector VIP (purple) or Vector Blue (L) as substrate. Bars represent 50 μm.
