Fig 2.
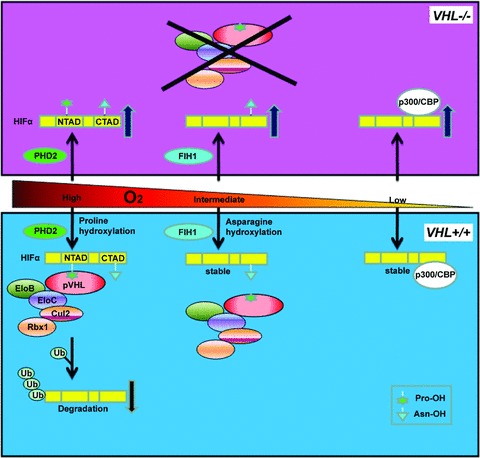
pVHL controls HIF via oxygen-sensitive hydroxylation. HIFα subunits have both an NTAD and a CTAD transactivation domain. When O2 levels are high, HIFα is hydroxylated on one or both conserved prolyl (Pro) residues located within the NTAD by the oxygen-dependent PHD2. This prolyl hydroxylation event generates a high affinity binding site for the pVHL E3 ubiquitin ligase complex composed of Cullin 2 (Cul2), Elongin B (EloB), Elongin C (EloC) and Rbx1. The pVHL complex polyubiquitinates HIFα, leading to its destruction by the proteasome. When O2 levels are intermediate, HIFα is hydroxylated by factor inhibiting HIF (FIH1) at a conserved asparaginyl (Asn) residue located in the CTAD, inhibiting HIF's interaction with the transcriptional co-activators p300/CBP. When O2 levels are low, HIFα subunits are stabilized, able to heterodimerize with the constitutively stable HIFβ, interact with p300/CBP and promote the transcription of downstream target genes. In cells lacking VHL, PHD2 and FIH1 remain active but HIFα subunits are not polyubquitinated and therefore allowed to accumulate.
