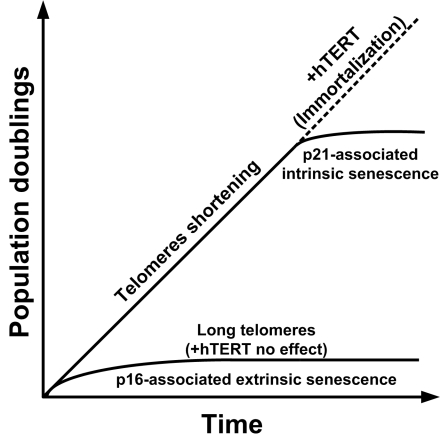
Figure 1. Mechanisms of cellular senescence in human cells.
In normal proliferating human cells, telomeres at the end of chromosomes are shortened at every cell division unless the cells express telomerase. When telomeres get too short, genomic instability ensues and a DNA-damage response under the control of the p21 pathway is induced. This causes growth arrest and intrinsic cellular senescence. Transduction of these senescence cells with a telomerase construct reverses this growth arrest and leads to immortalization. When cells undergo stress (e.g. reactive oxygen species, ionizing radiations, etc.) they can undergo p16-mediated extrinsic senescence even though they possess long telomeres. Re-expression of telomerase in this case does not rescue this irreversible growth arrest. Murine cells have very long telomeres and are not thought to be susceptible to intrinsic senescence in normal conditions. However, they are very sensitive to extrinsic senescence. Murine cells often escape from p16-mediated senescence and get immortalized but the mechanism for this is unclear.