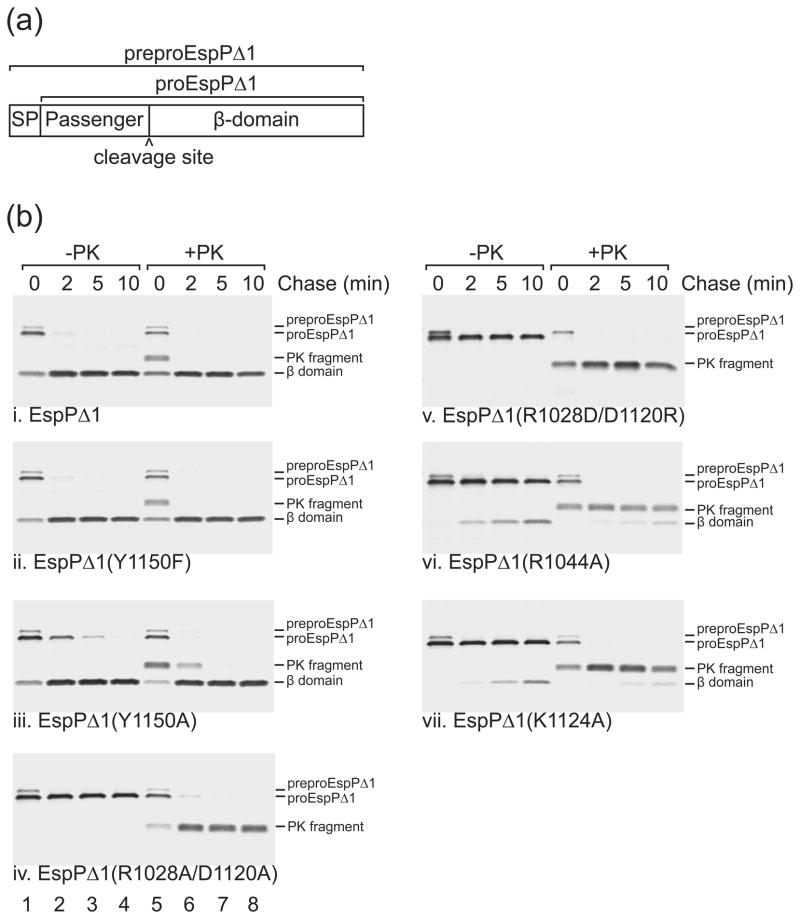
Fig. 3. Effects of mutations near the EspP cleavage site on passenger domain cleavage.
(a) Diagram showing EspPΔ1, a truncated form of EspP that contains the C-terminal 116 residues of the passenger domain followed by the β-domain. The construct that we used also contains the OmpA signal peptide (SP) to target EspPΔ1 to the Sec machinery. The signal peptide of preproEspPΔ1 is removed during its translocation across the inner membrane to generate proEspPΔ1. Following translocation of the passenger domain across the outer membrane, proEspP is cleaved into discrete passenger domain and β domain fragments (b) AD202 expressing espPΔ1 or the indicated mutant were subjected to pulse-chase labeling and proteinase K (PK) was added to half of each sample. Immunoprecipitations were then conducted with an antiserum directed against a C-terminal EspP peptide. The length of the chase is indicated. Lanes 1–4, no PK added (−PK); lanes 5–8, PK added (+PK).