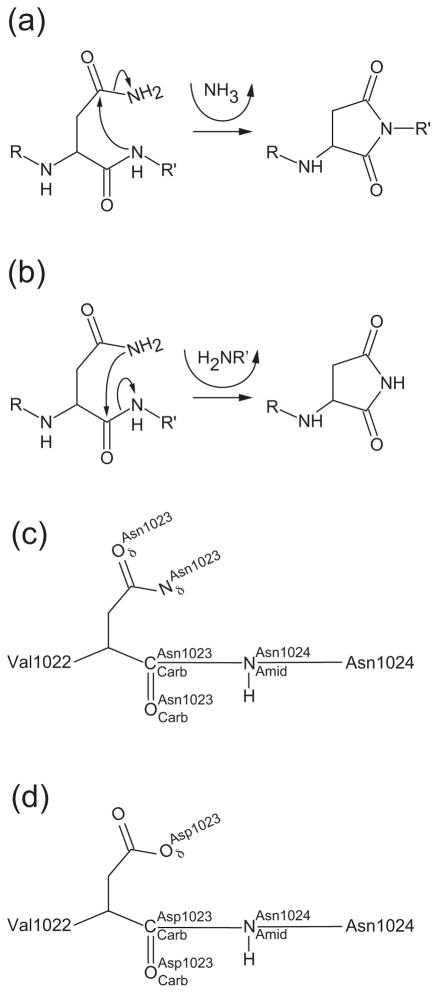
Fig. 4. Consequences of asparagine cyclization.
(a) Deamidation pathway. The peptide bond nitrogen attacks the carboxamide group of the upstream asparagine residue resulting in the formation of a succinimide ring in the protein backbone and production of ammonia. (b) Cleavage pathway. The side chain nitrogen of asparagine attacks its own main chain carbonyl carbon resulting in cleavage of the peptide bond. A succinimide ring is formed at the new C-terminus. The new N-terminus begins with the residue directly downstream of the active site asparagine. (c and d) Nomenclature used in text for atoms involved in asparagine cyclization. (c) Wild-type asparagine shown at position 1023 or (d) substituted aspartate shown at position 1023.