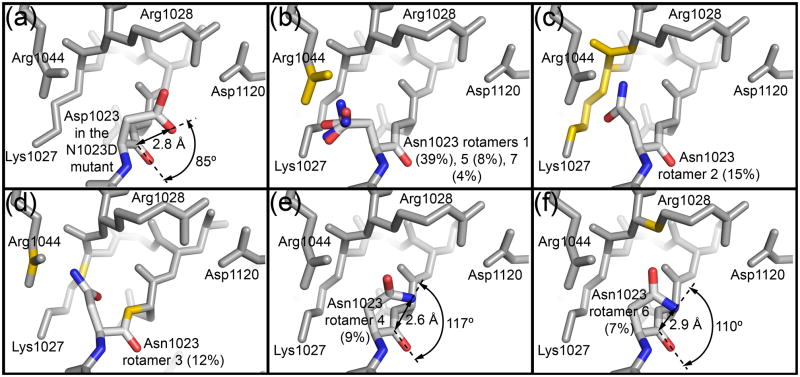
Fig. 5. Steric constraints limit the active site asparagine to rotamers favorable for cyclization.
(a) Burgi-Dunitz angle for Asp1023 in the EspP N1023D mutant. (b–f) The seven most common rotamers for asparagine from the Coot library were substituted at position 1023 in the EspP N1023A mutant to create the models. Each rotamer’s percentage abundance is shown in parentheses. Side chain and main chain atoms that clash with the asparagine rotamers are shown in yellow. For Panel (b), three similar rotamers (1,5,7) are shown. Rotamers 4 and 6 (Panels e and f) show the least clashes. When their carboxamide groups are rotated 180° such that is oriented towards , they are in a reasonable position for cyclization. The distance between and and the Burgi-Dunitz angle are shown for each of these rotamers.