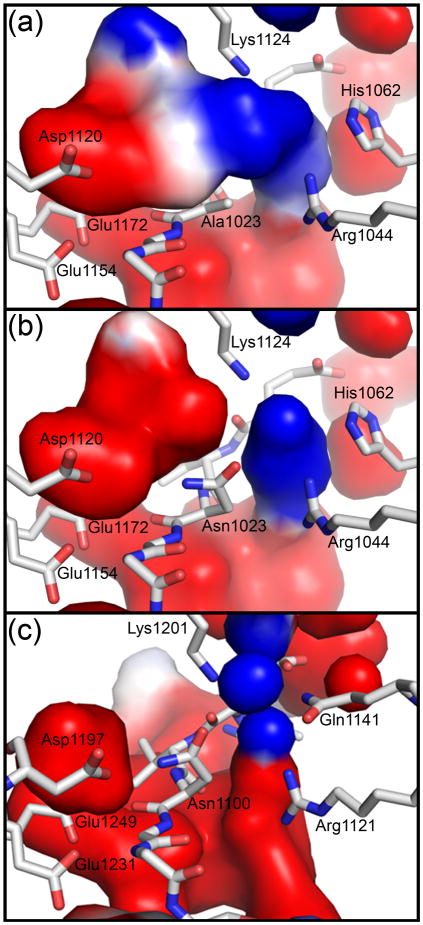
Fig. 6. Electrostatic interactions position the carboxamide group of the active site asparagine for cyclization.
The electrostatic surfaces were calculated using APBS tools (red, negative; blue, positive; white, neutral) 41. (a) A region of negative charge and a region of positive charge are on either side above Ala1023 in the EspP N1023A mutant. (b) The asparagine rotamer seen in Fig. 5e was substituted at position 1023 of the EspP N1023A mutant and the electrostatic surfaces recalculated. The resulting surfaces would likely orient the carboxamide group of Asn1023 such that would be placed near and in position for cyclization. (c) The asparagine rotamer seen in Fig. 5e was substituted at position 1100 of the Hbp N1100D mutant. The electrostatic surfaces are similar to those seen for EspP and would be expected to correctly orient the carboxamide group of Hbp Asn1100 for cyclization.