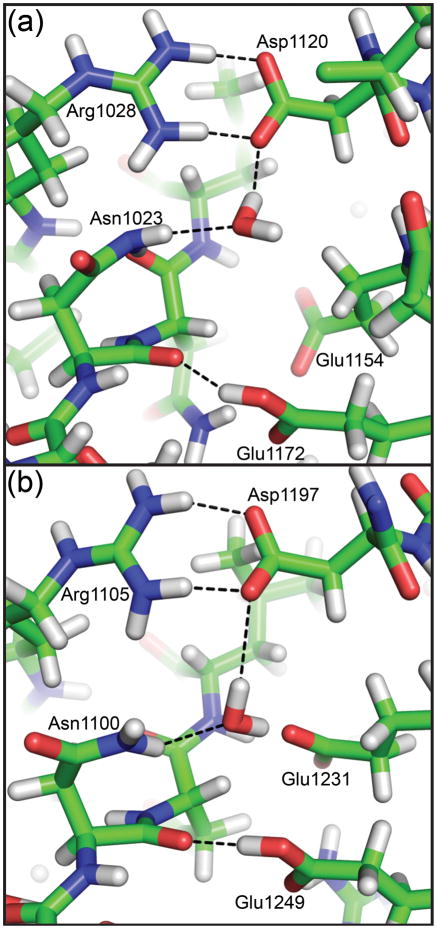
Fig. 7. Representative snapshots of the cleavage sites of EspP and Hbp from molecular dynamics simulations.
Modeling asparagine at position 1023 of the EspP N1023A mutant (Panel (a)) or 1100 of the Hbp N1100D mutant (Panel (b)) created the wild-type models. A water molecule is shown for EspP and Hbp that forms hydrogen bonds with the active site asparagine and EspP Asp1120 or Hbp Asp1197. These hydrogen bonds would be favorable for increasing the nucleophilicity of the active site asparagine. All hydrogen bonds shown are less than 3.5 Å when measured between non-hydrogen atoms. The water molecule in Panel (a) is also within 3.5 Å of EspP Arg1028 and the water molecule in Panel (b) is within 3.5 Å of Hbp Glu1231 and Glu1249.