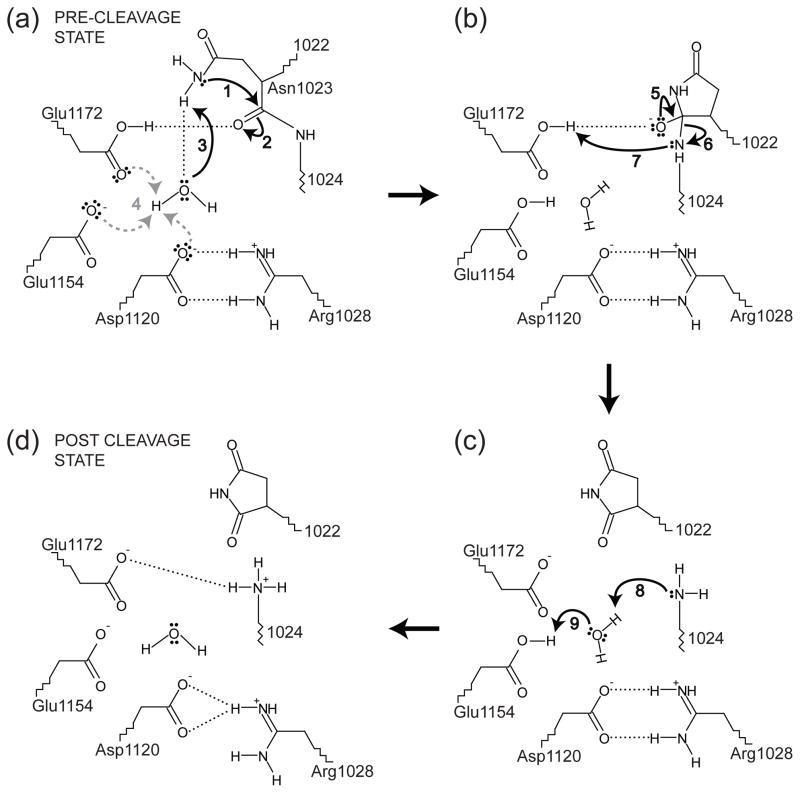
Fig. 8. Proposed mechanism for EspP passenger domain cleavage.
The residues shown are labeled using EspP numbering and the mechanism is described in the main text. In Panel (a), the water molecule hydrogen bonding to could be positioned by hydrogen bonding to Asp1120, Glu1154, Glu1172, or a combination these residues. Additionally, the proton from that is absorbed by the putative catalytic water in panel (a) could result in a proton being transferred to Asp1120, Glu1154 or Glu1172 (dashed, gray arrows). For simplicity, this proton is shown being transferred to Glu1154 in panels (b) and (c). In Panel (d) the hydrogen bonding pattern matches the post cleavage structure of EspP. The water shown in Panel (d) is not seen in the post cleavage structure. However, only six waters were included in the post-cleavage structure due to the resolution of the data.