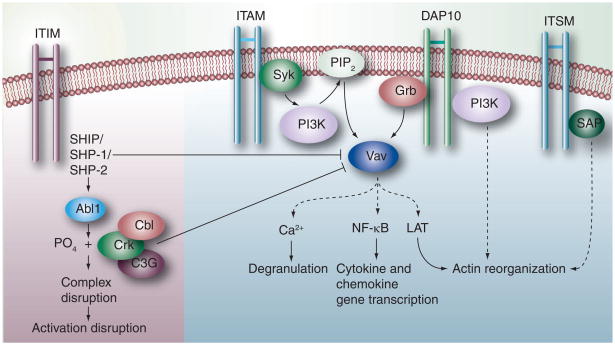
Figure 2. NK signaling cross-talk.
Upon engagement of a ligand by the inhibitory receptor, the tyrosine residue of inhibitory motif ITIM is phosphorylated so as to recruit the lipid phosphatase SHIP or the tyrosine phosphatase SHP-1 or SHP-2. These dephosphorylate Vav of the activation pathway and block the subsequent signaling for activation. Alternatively, phosphorylation of the adaptor protein Crk by Abl1 disrupts the activation complex. Cbl may also inhibit phosphorylation of Vav leading to a blockade of the activation pathways. The ITAM-containing residues are phosphorylated by the Src family protein tyrosine kinases to bind Syk and ZAP tyrosine kinases. At this point, intracellular signaling molecules such as PLC-γ and the nucleotide exchange factors of the Vav family are recruited by NK cells depending on their stage of maturation. A cascade of reactions follows which induces degranulation using Ca2+ ions, NF-κB for transcription of cytokine and chemokine genes or LAT-mediated actin reorganization and degranulation. The recruitment of Grb to the DAP10 signaling motif triggers the downstream phosphorylation of Vav1, ultimately leading to actin reorganization and degranulation through a series of reactions. The ITSM of the CD244 receptor molecules is recognized by SAP (SH2D1A), which is a cytoplasmic SH2 domain-containing adaptor protein. Tyrosine phosphorylation of the ITSM by SAP leads to the activation of NK cells via a series of reactions.
ITAM: Immunoreceptor tyrosine-based activation motif; ITIM: Immunoreceptor tyrosine-based inhibitory motif; ITSM: Immunoreceptor tyrosine-based switch motif; LAT: Linker of activated T cell.