Abstract
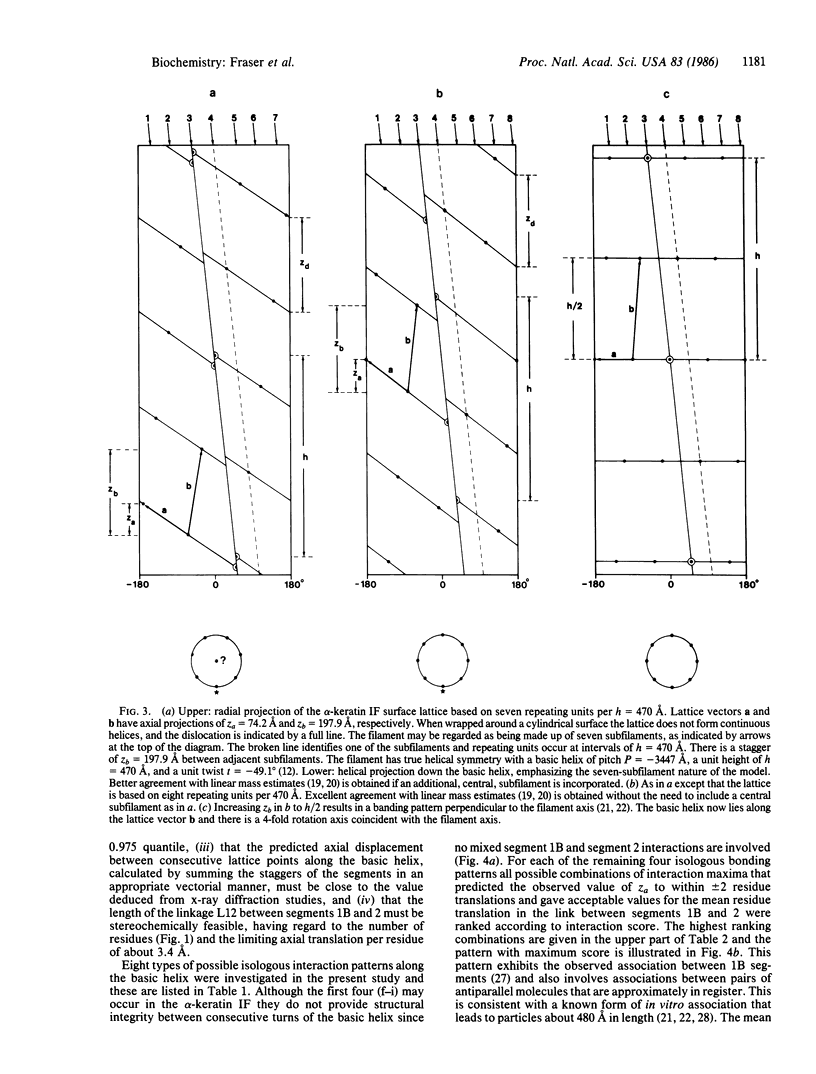
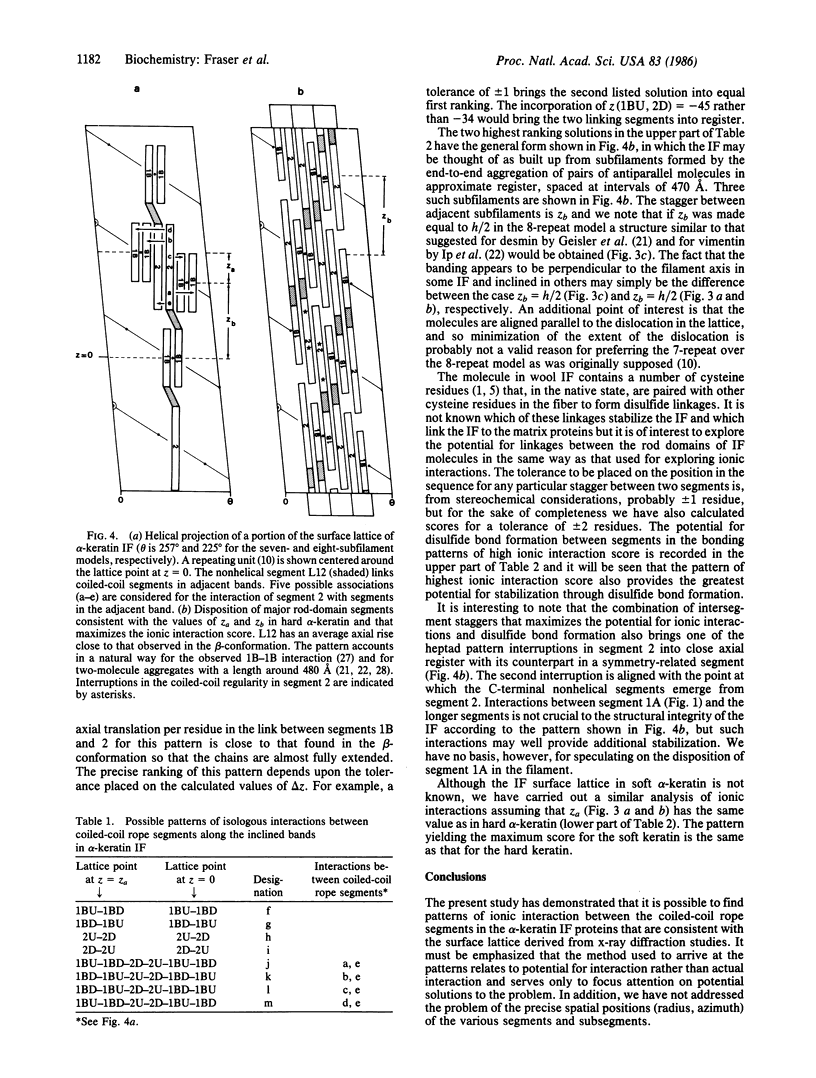
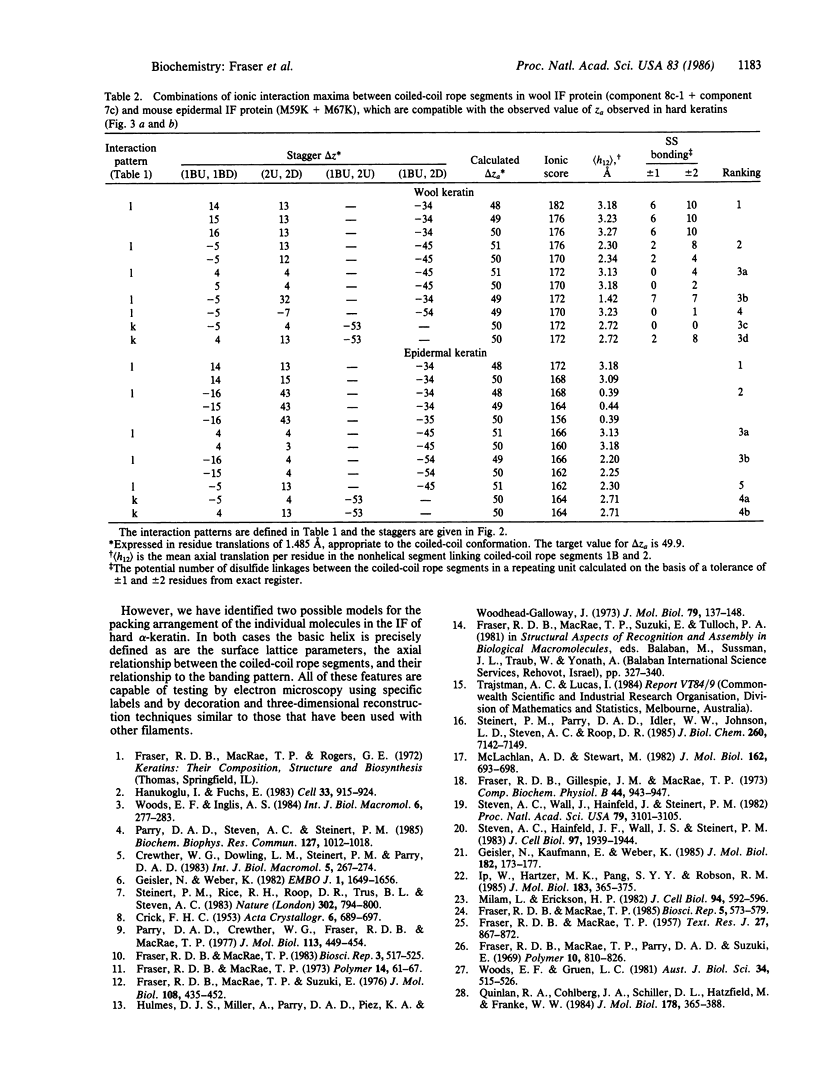
Previous x-ray diffraction studies on the alpha-keratins of hair and wool have revealed that the intermediate filaments (IF) have a helical structure rendered imperfect by a precisely defined dislocation. It has also been possible to deduce a surface lattice for the IF and to determine the number of IF molecules associated with each lattice point. In this work this information is combined with data on the ionic interactions between the coiled-coil rope segments of the IF molecules to provide a plausible model for the pattern of interactions that stabilize the framework of the IF in the "hard" alpha-keratins. Similar interaction studies of the proteins from the IF in the so-called "soft" alpha-keratin from the stratum corneum layer of the skin suggest that they are likely to have an essentially similar pattern.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fraser R. D., Gillespie J. M., Macrae T. P. Tyrosine-rich proteins in keratins. Comp Biochem Physiol B. 1973 Mar 15;44(3):943–947. doi: 10.1016/0305-0491(73)90244-7. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P. Intermediate filament structure. Biosci Rep. 1985 Jul;5(7):573–579. doi: 10.1007/BF01117070. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Suzuki E. Structure of the alpha-keratin microfibril. J Mol Biol. 1976 Dec;108(2):435–452. doi: 10.1016/s0022-2836(76)80129-5. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P. The structure of the alpha-keratin microfibril. Biosci Rep. 1983 Jun;3(6):517–525. doi: 10.1007/BF01120695. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Antiparallel orientation of the two double-stranded coiled-coils in the tetrameric protofilament unit of intermediate filaments. J Mol Biol. 1985 Mar 5;182(1):173–177. doi: 10.1016/0022-2836(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A., Parry D. A., Piez K. A., Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol. 1973 Sep 5;79(1):137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- Ip W., Hartzer M. K., Pang Y. Y., Robson R. M. Assembly of vimentin in vitro and its implications concerning the structure of intermediate filaments. J Mol Biol. 1985 Jun 5;183(3):365–375. doi: 10.1016/0022-2836(85)90007-5. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Periodic charge distribution in the intermediate filament proteins desmin and vimentin. J Mol Biol. 1982 Dec 15;162(3):693–698. doi: 10.1016/0022-2836(82)90396-5. [DOI] [PubMed] [Google Scholar]
- Milam L., Erickson H. P. Visualization of a 21-nm axial periodicity in shadowed keratin filaments and neurofilaments. J Cell Biol. 1982 Sep;94(3):592–596. doi: 10.1083/jcb.94.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Steven A. C., Steinert P. M. The coiled-coil molecules of intermediate filaments consist of two parallel chains in exact axial register. Biochem Biophys Res Commun. 1985 Mar 29;127(3):1012–1018. doi: 10.1016/s0006-291x(85)80045-0. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Cohlberg J. A., Schiller D. L., Hatzfeld M., Franke W. W. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984 Sep 15;178(2):365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A., Idler W. W., Johnson L. D., Steven A. C., Roop D. R. Amino acid sequences of mouse and human epidermal type II keratins of Mr 67,000 provide a systematic basis for the structural and functional diversity of the end domains of keratin intermediate filament subunits. J Biol Chem. 1985 Jun 10;260(11):7142–7149. [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Hainfeld J. F., Trus B. L., Wall J. S., Steinert P. M. Epidermal keratin filaments assembled in vitro have masses-per-unit-length that scale according to average subunit mass: structural basis for homologous packing of subunits in intermediate filaments. J Cell Biol. 1983 Dec;97(6):1939–1944. doi: 10.1083/jcb.97.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Wall J., Hainfeld J., Steinert P. M. Structure of fibroblastic intermediate filaments: analysis of scanning transmission electron microscopy. Proc Natl Acad Sci U S A. 1982 May;79(10):3101–3105. doi: 10.1073/pnas.79.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods E. F., Gruen L. C. Structural studies on the microfibrillar proteins of wool: characterization of the alpha-helix-rich particle produced by chymotryptic digestion. Aust J Biol Sci. 1981;34(5-6):515–526. doi: 10.1071/bi9810515. [DOI] [PubMed] [Google Scholar]



