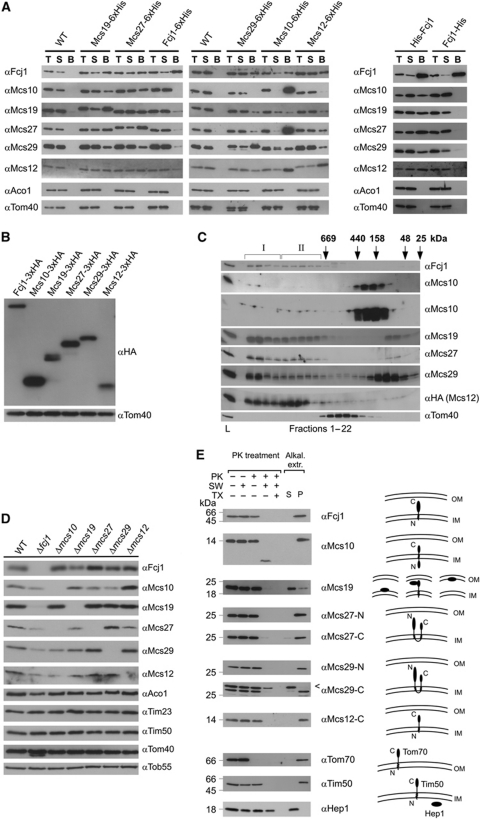
Figure 2.
The MICOS complex. (A) Co-isolation of Mcs proteins. His-tagged versions of the Mcs proteins were expressed under control of their own promoters (left panel) or from the pYX242 plasmid (right panel) in the respective deletion strain. Mitochondria were isolated, solubilized with digitonin and incubated with Ni-NTA beads. Total (T, 5% of total), supernatant (S, 5% of total) and bound material (B, 100%) were analysed by SDS–PAGE and immunodecoration with the indicated antibodies. Mitochondria from wild-type cells served as control. (B) Relative abundance of Mcs proteins. HA-tagged versions of the Mcs proteins were expressed under their own promoters. Equal amounts of mitochondria were analysed by SDS–PAGE and immunoblotting with antibodies against the HA-tag (αHA) and against Tom40 (αTom40), the loading control. (C) Molecular sizing of Mcs proteins from wild-type mitochondria. Mitochondria were lysed with digitonin and lysates subjected to gel filtration on a Superose 6 column. The fractions were analysed by SDS–PAGE and immunoblotting using the indicated antibodies; in case of Msc12, a strain was used which expressed HA-tagged Msc12 and immunoblotting was with αHA antibody. Msc10 blots were exposed for two different time periods. The TOM complex (Tom40) was decorated as a control. I and II, MICOS complex I and II. Positions of marker proteins for calibration are indicated with arrows. L, load (10% of material applied to column). See also Supplementary Figure S2A. (D) Steady-state levels of Mcs proteins in cells in which one of the MCS genes was deleted. Mitochondria were analysed by SDS–PAGE and immunoblotting. See also Supplementary Figure S2B. (E) Membrane integration and orientation of Mcs proteins. (Left) Mitochondria from wild-type cells were left untreated or treated with proteinase K (PK) either directly, after subjecting them to osmotic swelling (SW) or after lysis with Triton X-100 (TX); bars indicate the apparent molecular masses of the full-length proteins. (Right) Mitochondria were exposed to alkaline extraction at pH 12. Soluble (S) and membrane integrated (P, pellet) material were separated by centrifugation. Aliquots were subjected to SDS–PAGE and immunodecoration with antibodies against the indicated proteins. Arrowhead, unspecific cross-reaction.