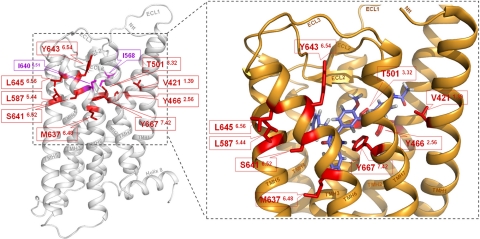
Figure 2.
Newly identified CAMs in the thyrotropin receptor surround the allosteric binding site of a LMW agonist. Left panel: molecular homology model of the serpentine domain (aa C408–I698) without the large N-terminal tail of the human TSHR is based on the crystal structure of inactive bovine rhodopsin. Backbone (white) of the TSHR and the previously identified CAMs (magenta) I640V (TMH6) and I568V (ECL2) are displayed. Nine positions of the newly identified CAMs (red sticks) are localized in the upper part of the TMHs toward the extracellular loops. Right panel: closeup view of the activated TSHR conformation, which is based on the opsin crystal structure, occupied by LMW-Ag (11). Wild-type amino acids of the 9 CAM positions (red) are arranged in a cluster. They are spatially close or interact directly with each other and surround the allosteric ligand binding pocket of LMW-Ag (blue). Therefore, these amino acids may be involved in TSHR activation by small-molecule ligands.