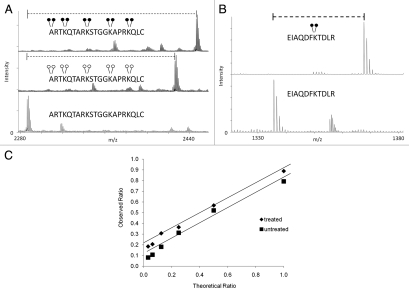
Figure 2.
Efficiency and dynamic range of MassSQUIRM. (A) A synthetic peptide containing four unmodified lysine residues (lower part) was exposed to reductive methylation using either light formaldehyde (middle part) or heavy formaldehyde (top part). The resulting spectra show a ∼100% conversion of all lysine residues, as well as the N-terminus, to the di-methyl state. Heavy formaldehyde showed a peak 20 Da larger than that of the light formaldehyde as would be expected. (B) MassSQUIRM is efficient in-gel with full-length proteins. A recombinant version of full-length human H3.2 was subjected to in-gel tryptic digestion either with (top part) or without (bottom part) prior in-gel isotopically heavy reductive methylation. Resulting peptides were analyzed by MALDI-TOF mass spectrometry. A ∼100% conversion to heavy di-methylated peptide was observed. (C) Un-, mono- and di-methylated synthetic peptides were normalized to a 1:1:1 mixture and mono-methylated peptide concentration was varied at the theoretical ratios indicated (untreated sample). The same peptide mixtures were separately treated with MassSQUIRM (treated sample). The linear dynamic range was determined to be 1:8 for both treated (◆) and untreated (■) samples. Open circles indicate light methylation while closed circles indicate heavy methylation.