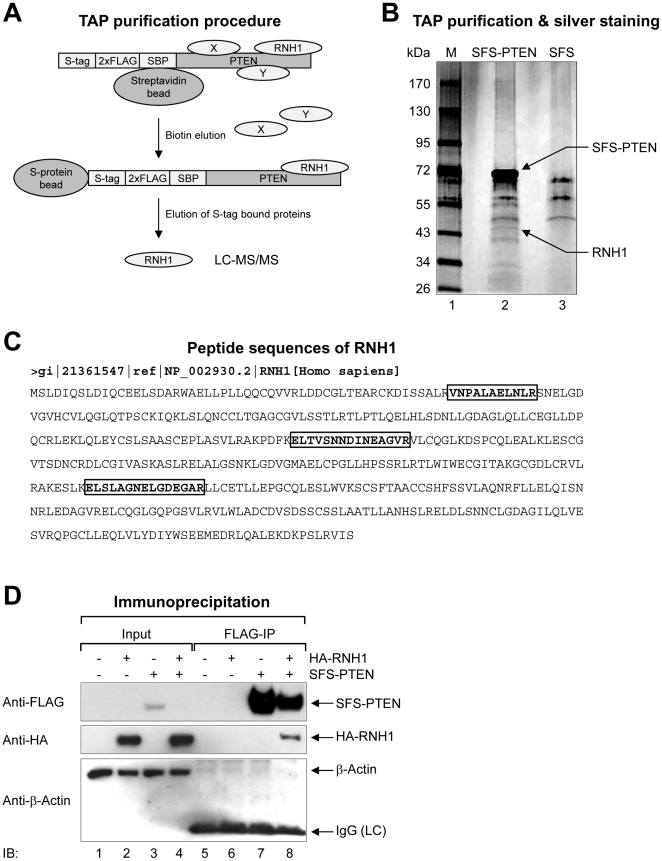
Figure 3. Identification of RNH1 as a novel PTEN-interacting protein.
(A) Schematic diagram of tandem affinity purification (TAP) procedure. A S-tag, double FLAG tag, and streptavidin-binding peptide were fused at the N-terminus of PTEN (SFS-PTEN). After sequential streptavidin and S-protein bead binding, the PTEN-interacting proteins were eluted from S-protein beads. The eluted proteins were analyzed by LC-MS/MS. X and Y represent nonspecifically interacting proteins. (B) The silver staining result of TAP purified proteins. Arrows indicate an RNA-regulatory protein RNH1 (lower) and SFS-PTEN (upper). (C) Peptide sequences (boxes) of RNH1 by LC-MS/MS analysis. (D) Physical interaction between PTEN and RNH1. Plasmids encoding HA-RNH1 and SFS-PTEN were ectopically expressed in 293T cells. SFS-PTEN was precipitated with anti-FLAG M2 affinity agarose gels and then immunoblotting with anti-FLAG, anti-HA, or anti-β-Actin (negative binding control) was performed. IgG (LC): IgG (light chain).