Figure 3.
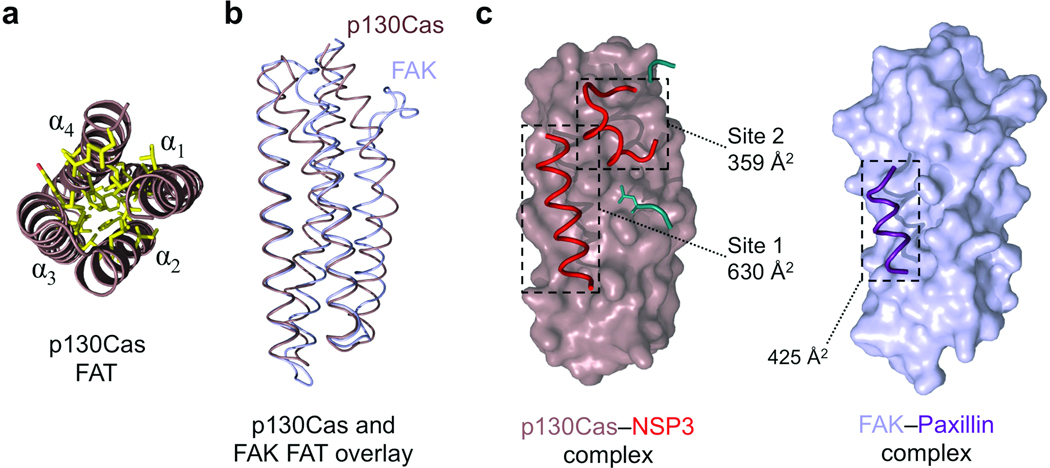
The FAT domain of p130Cas is well defined and employs a novel extended binding mode. (a) The p130Cas FAT domain core. End-on view of the four helical bundle of p130Cas (brown) showing a close knit hydrophobic network (yellow) stabilizing the core of the helical arrangement. (b) Backbone overlay of p130Cas (brown) with FAK (light blue, from PDB 1ow7)30. (c) The interaction mode used by the FAT domain of p130Cas and NSP3 compared to FAK binding to the Paxillin LD-4 peptide (purple). NSP3 elements contributing to the Site 1 and Site 2 binding motifs are colored red. Buried surface areas for NSP3–p130Cas Site 1, Site 2, and FAK–Paxillin are indicated. Additional NSP3 residues participating in the Cas interaction are colored cyan, and add an extra 233 Å2 in buried surface area (not annotated).