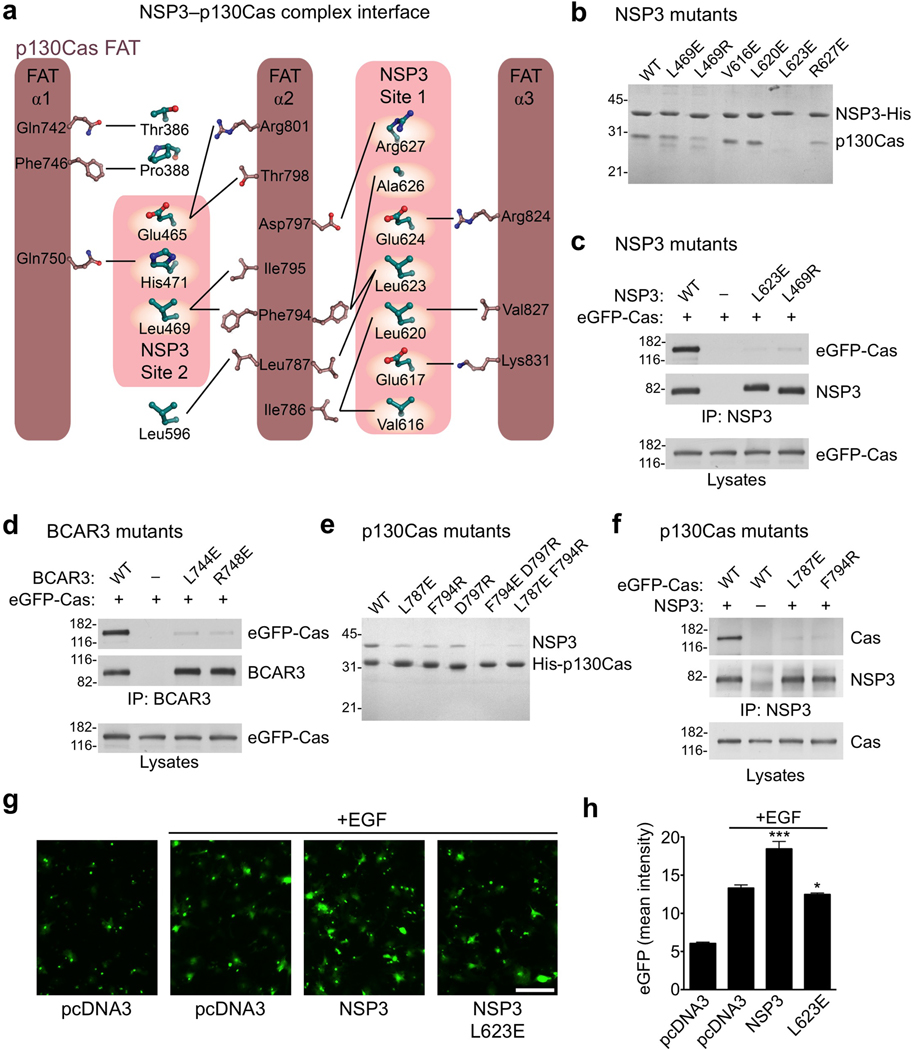
Figure 4.
Analysis of NSP family-p130Cas interactions in vitro and in vivo. (a) Schematic representation of the interfaces between NSP3 and p130Cas. (b) A subset of well-expressed NSP3 mutants (Cdc25-homology domain) were purified as His6-tagged proteins and assayed for their ability to selectively co-purify untagged WT p130Cas C-terminal domain from E. coli lysates. (c) NSP3 (full-length) mutants L623E and L469R show almost complete loss of p130Cas (full-length) binding, as shown following co-immunoprecipitation with an anti-NSP3 antibody. (d) Residues L744E and R748E in BCAR3 (equivalent to NSP3 residues Leu623 and Arg627 respectively) were examined for interaction with Cas following immunoprecipitation with an anti-BCAR3 antibody. Their near complete loss of p130Cas association in COS cells is in agreement with a conserved mode of binding. (e) Co-purification of recombinant p130Cas mutants with NSP3, performed as shown in panel b, but using p130Cas with a His6–tag and untagged NSP3. (f) p130Cas mutants were examined for their ability to bind NSP3 in COS cells by immunopreciptation with an anti-NSP3 antibody. (g,h) NSP3–Cas interaction promotes chemotaxis towards an EGF chemoattractant in transwell migration assays. (g) Representative images of cells that relocated from the upper to the lower side of the Transwell filter (scale bar, 100 µm). (h) Histogram quantifying the mean eGFP intensity from transfected cells on the lower side of the filters. Error bars indicate the standard deviation from six microscope field measurements. (* P<0.05 and *** P<0.001)