Figure 8.
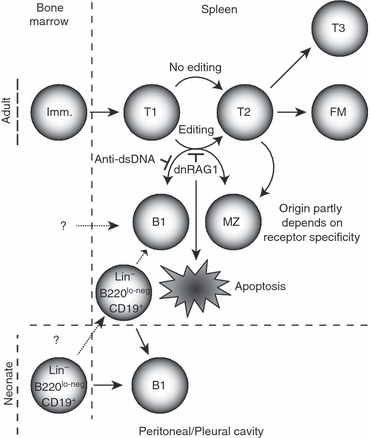
Model for receptor editing's role in guiding B-cell differentiation. Non-self-reactive immature B cells arriving from the bone marrow progress normally through the transitional stages of B-cell development. However, those arriving with receptor specificities tolerated in the host, but normally edited in the adult spleen at the T1 stage, are prevented from editing by dnRAG1 expression. As a result, B cells may acquire a B1 or marginal zone (MZ) phenotype; those with anti-dsDNA specificity preferentially acquire the MZ phenotype. Alternatively, B cells retaining a self-specificity that is not tolerated in the periphery may undergo apoptosis. T2 B cells not subjected to editing (or having successfully edited) may also differentiate into MZ B cells as discussed previously.61 Even if receptor editing is impaired in the conventional B2 lineage, peritoneal B1 B cells arising from a specified Lin− B220lo-neg CD19+ progenitor may continue to persist.54,59 It is also possible that a different haematopoietic progenitor cell uniquely gives rise to a splenic B1 cell population that is distinct from B1-like cells that acquire this phenotype in response to a failed editing programme.
