Abstract
BACKGROUND AND PURPOSE
Tianeptine is an antidepressant affecting the glutamatergic system. In spite of its proven clinical efficacy, molecular effects of tianeptine are not entirely clear. Tianeptine modulates cytokine expression in the CNS and protects the hippocampus from chronic stress effects. HIV infection is associated with inflammation and neuronal loss, causing HIV-associated dementia (HAD). The human immunodeficiency virus type-1 glycoprotein gp120 has been proposed as a likely aetiological agent of HAD. In this study, we determined whether tianeptine protects astroglial cells from the neurodegenerative effects of gp120.
EXPERIMENTAL APPROACH
Human astroglial cells were treated with gp120 and tianeptine, and viability and apoptosis was monitored by TUNEL, annexin V, and activated caspase-3 staining and flow cytometry. Protein levels of glutamine synthase (GS), inducible and constitutive nitric oxide synthases (iNOS, cNOS) and nuclear factor κB (NF-κB) pathway were determined by Western blot analysis. The respective activities were assessed indirectly by measuring glutamine and nitrite concentrations or by luciferase reporter assays.
KEY RESULTS
Tianeptine showed an anti-apoptotic effect and prevented caspase-3 activation by gp120. The mechanism of tianeptine's action involved GS and cNOS stabilization and iNOS suppression. Moreover, tianeptine increased IκB-α levels in the absence of gp120 and blocked its degradation in response to gp120. This correlated with the suppression of basal and gp120-induced NF-κB transcriptional activity.
CONCLUSIONS AND IMPLICATIONS
Tianeptine clearly exerts neuroprotective effects in vitro by suppressing the molecular pro-inflammatory effects of gp120. Studies in animal models should be performed to evaluate the potential of tianeptine as a treatment for HAD.
Keywords: molecular pharmacology, nitric oxide, antidepressant drugs, neuroprotective drugs, apoptosis, inflammation
Introduction
Tianeptine is an antidepressant with a unique mode of action. It interferes with the effects of stress on glutamatergic transmission (Kole et al., 2002; McEwen and Chattarji, 2004; McEwen and Olie, 2005; Reagan et al., 2004; 2007; Spedding and Lestage, 2005; Svenningsson et al., 2007) and represents one of the few agents which do not inhibit monoamine uptake. Paradoxically, tianeptine has been reported to enhance 5-HT re-uptake, in contrast to the majority of antidepressive drugs which act as selective 5-HT (serotonin) re-uptake inhibitors, although this action might be secondary to other effects induced by tianeptine (McEwen and Olie, 2005).
Evidence has accumulated showing that tianeptine acts via modulation of glutamatergic transmission and glutamate turnover. Tianeptine induces serine phosphorylation of AMPA receptors and this effect positively regulates glutamate transmission and plasticity at excitatory synapses (Svenningsson et al., 2007). This molecular mechanism may explain many of the biological effects of tianeptine, such as the inhibition of stress-induced reduction in cell proliferation and hippocampal volume, stress-dependent activation of the amygdale as well as the decrease in cerebral metabolites elicited by chronic stress (Czeh et al., 2001; Fuchs et al., 2004; McEwen and Chattarji; 2004; Reagan et al., 2007). Besides the induction of AMPA receptor phosphorylation, tianeptine has been shown to inhibit chronic restraint stress-induced changes in glutamate transporter EAAT2 (GLT1) expression on glial cells. Interestingly, tianeptine administration to control rats had no effect on EAAT2 and this suggests that tianeptine may act to normalize glutamatergic function during stressful stimuli, an effect which might involve some complex regulatory mechanisms occurring within astrocytes (Reagan et al., 2004).
Evidence exists that the conversion of glutamate to glutamine, taking place within the astrocytes, represents a key mechanism in the regulation of excitatory neurotransmission under normal conditions as well as in injured brain (Szatkowski and Attwell, 1994). The synaptically released glutamate is taken up by astroglial cells and then converted into non-toxic glutamine by the glia-specific enzyme glutamine synthase (GS); on the other hand, glutamine re-enters the glutamatergic neurone where it is converted by glutaminase into glutamate, thus replenishing the neurotransmitter pool (Kennedy et al., 1974). The possible modulation of GS activity has been studied during numerous neuropathological states, including inflammation, ischaemia/reperfusion injury, etc. (Oliver et al., 1990; Huang and O'Banion, 1998). In particular, overproduction of reactive oxygen species, which occurs during excitotoxicity in brain tissues, leads to a reduced ability of astroglial cells to regulate glutamate turnover via inhibition of GS activity (Butterfield et al., 1997). Moreover, the peroxynitrite, generated by the reaction between NO and superoxide anions, leads to nitration of tyrosine residues located at the active site of GS enzyme (Muscoli et al., 2007). Thus, both oxygen- and nitrogen-reactive species interfere with GS activity in astrocytes.
We have recently demonstrated that inflammatory stimuli such as lipopolysaccharide (LPS) plus interferon γ (INF-γ) as well as the HIV-coating gp120 induce GS dysregulation in cultured astrocytes (Muscoli et al., 2005; Visalli et al., 2007). Interestingly, tianeptine has been shown to attenuate the effects of LPS on the CNS by decreasing the production of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and INF-γ, and inducing anti-inflammatory cytokines such as IL-10 (Castanon et al., 2001; 2003; 2004;). Moreover, tianeptine prevents the IL-1β-induced glutamatergic lesions in the cortex and white matter (Plaisant et al., 2003). The mechanism underlying these anti-inflammatory effects of tianeptine has not been addressed so far. As the effects of LPS seem to involve cross-talk between inducible and constitutive release of nitric oxide (NO) and activation of nuclear factor κB (NF-kB) pathway (Colasanti et al., 1997), it is likely that the amount of NO released under different pathophysiological conditions may represent a crucial mechanism affecting GS activity and glutamate turnover in glial cells.
The experiments reported in this work have been designed to study: (i) the effect of tianeptine on HIV coating gp120-related changes of GS activity and expression in human cultured astroglial cells; (ii) possible modulation of NOS by tianeptine in astrocytes undergoing gp120-related injury; and (iii) possible mechanisms of this modulation. We show that the powerful protective action of tianeptine is accompanied by an increase in GS activity, an effect which is likely to be secondary to the modulation of constitutive and inducible forms of NOS and to the repression of the NF-κB pathway.
Methods
Cell culture
The astroglial cell lines Lipari and U373 were prepared and grown as previously described (Mollace et al., 2002). The majority of the experiments were performed on Lipari astroglial cells unless indicated otherwise. Cells were expanded and cultured by seeding them in 10 cm plastic dishes at a density of 2 × 106 cells per dish in DMEM supplemented with 10% fetal calf serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin (complete medium), and incubated at 37°C in humidified air containing 5% CO2. For all experiments the cells were seeded at 0.8 × 104 per cm2 the day before the treatments or otherwise indicated.
Reagents and antibodies
Tianeptine (Servier, Neuilly-sur-Seine, France) was freshly prepared for each experiment as 10 mM stock solution in Dulbecco's modified Eagle's medium (DMEM). Recombinant HIV-1 (IIIB) and glycosylated gp120 with a molecular weight of approximately 115 kDa, produced using the baculovirus expression system (purity: >90% by SDS-PAGE) was purchased from RDI Division (Fitzgerald Industries International, Acton, MA, USA). The following antibodies (Abs) were purchased from Transduction Laboratories (Lexington, KY, USA) and used for Western blotting: monoclonal anti-glutamine synthase (cat. 645020, used 1:3000), monoclonal anti-iNOS (cat. N39120, used 1:2000), monoclonal anti-nNOS (cat. 610427, 1:2000). Other Abs used for Western blotting were: anti-IκB-α (SC-1643, used 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal anti-β-actin (1:5000; Sigma-Aldrich, St Louis, MO, USA).
Flow cytometric analysis of Annexin V positive cells
The supernatant medium from astroglial cells grown on 24 well plates and treated for the required time with tianeptine and gp120 was collected in flow cytometry tubes. Adherent cells were detached by trypsin and mixed with the respective supernatants, washed with phosphate buffered saline (PBS) and resuspended in PBS, containing 1% fetal bovine serum (FBS) at 5 × 105 mL−1 cell density. Then 100 µL of cells were mixed with 100 µL of Binding buffer (BioVision, cat. 1035–100; Mountain View, CA, USA) containing 7-amino actinomycin (7-AAD) and 2 µL of FITC-Annexin V (BioVision, cat. 1001–200) and incubated according to the manufacturer's instructions. Annexin V and 7-AAD positive cells were analysed by FACS Canto II Flow Cytometer (BD Biosciences, Erenbodgem, Belgium) in 530 and 630 nm channels (FITC and PerCP-Cy5 channels). All treatments and labelling were performed in triplicate.
Activated caspase-3 analysis by flow cytometry
After the various treatments (performed in 6 well plates), the astroglial cells were incubated in the pre-warmed DMEM (10% FBS) with 5 µM FITC-z-VAD-fmk (Promega, Madison, WI, USA) at 37°C for 20 min. Next, the cells were washed twice in PBS, trypsinized for 5 min and collected in flow cytometry tubes. The cells were pelleted by centrifuging for 4 min at 350×g, washed in PBS and finally suspended in PBS or PBS containing 1 µg·mL−1 propidium iodide. After 5 min, stained and control unstained cells (treated in parallel) were acquired at FACS Canto II Flow Cytometer with 530 and 585 nm channels. Analysis was performed by FlowJo software (Treestar Inc., Ashland, OR, USA). The propidium iodide positive cells were excluded from analysis.
Glutamine assay
Glutamine levels in the supernatants of human astroglial cells were determined by using a glutamine assay kit (Sigma-Aldrich) based on the reductive deamination of glutamine by a proprietary enzyme. The reaction is specific for glutamine and does not cross-react with other amino acids or ammonia. Briefly, cell supernatants, glutamine standards and cell culture medium were incubated with the reaction buffer, the dilution buffer and the specific enzyme for 1 h 30 min at 37°C. The colour reagent was added to each sample and the samples were allowed to stand for 5–10 min at room temperature. Absorbance was measured at 550 nm using a spectrophotometer. To calculate the quantity of glutamine, a linear regression analysis of the standard curve was performed. The GS-derived glutamine levels were considered as the amount of glutamine present above the basal levels of glutamine in the medium, incubated as cell cultures but without the cells.
Nitrite concentration measurements
Nitrite (NO2−) in the cell culture supernatant was measured by the Griess reaction. Aliquots of the cell supernatants were mixed with an equal volume of Griess reagent (1% sulphanilamide/0.1% naphlethylendiamine dihydrochloride/2.5% H3PO4). The absorbancy was measured at 546 nm and NO2− concentration was determined with sodium NO2− as a standard. Results are expressed as nmol NO2− mL−1 or normalized to the NO2− levels in control cells.
Western blotting analysis
Astroglial cells were seeded on 6 well plates at a density of 80–100 000 cells per well. Next day, the cell monolayers were either treated or untreated with gp120 and/or tianeptine for the required time periods. For NOS and GS Western blotting, the monolayers were washed once with PBS and solubilized by direct addition of a preheated (to 80°C) denaturing buffer, containing 50 mM Tris-HCl pH 6.8, 2% SDS and protease inhibitor cocktail (Sigma-Aldrich). Solubilized samples were collected and immediately boiled for 2 min. Bromophenol blue, glycerol and β-mercaptoethanol were then added to final concentrations of 0.05, 10 and 2% respectively. Samples were boiled again before being loaded onto 10% polyacrylamide SDS (SDS-PAGE) gels. For IκB-α analysis, the cells were lysed in cold radioimmunoprecipitation (RIPA) buffer (1% Igepal, 50 mM Tris-HCl ph 7.5, 150 mM NaCl, 1 mM EDTA) supplemented with protease (CompleteMini, Roche Diagnostics, Indianapolis, IN, USA) and phosphatase inhibitors (NaF 2 mM, sodium orthovanadate 1 mM); protein concentration was quantified by BCA protein assay (Pierce, Rockford, IL, USA); and lysates were processed by boiling for 5 min at 95°C in Laemmli buffer. Then 4 µg of protein lysate was loaded on 8% SDS-PAGE gels. After electrophoresis, polypeptides were electrophoretically transferred to nitrocellulose filters (Bio-Rad, Hercules, CA, USA). The incubation with the primary antibody was performed overnight in 5% milk. After incubation with secondary antibody (1:5000, anti-mouse horseradish peroxidase conjugate from Transduction Laboratories), blots were developed with the enhanced chemiluminescence procedure, using reagents (ECL-Plus) from Amersham Life Science (Piscataway, NJ, USA).
NF-κB promoter activity luciferase assay
Transcriptional activation of NF-κB was analysed by Dual luciferase assay system (Promega). U373 astroglial cells were seeded on 24 well plates and 24 h later the cells were transfected with reduced amounts of plasmids and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to minimize toxic effects of transfection. The following reporter plasmids were used: pNF-κB-Luc plasmid (2.25 µg per 24 wells), with the firefly luciferase gene under the control of five repeats of the NF-κB enhancer element (Stratagene, La Jolla, CA, USA). Transfection efficiency was normalized by co-transfecting cells (0.2 µg per 24 wells) with pRL-CMV vector (Promega), a plasmid encoding the renilla luciferase gene under CMV promoter. Transfection was carried for 2.5 h. 20 h after transfection the cells were stimulated with gp120 and/or tianeptine for 5 h. Finally, the cells were lysed and processed according to Promega kit instructions. Luciferase activities were measured by DCR-1 luminometer (Digene Diagnostics, Gaithersburg, MD, USA). Background values were adjusted with non-transfected lysates. Each lysate was measured at least three times and each treatment was performed six times.
Data analysis and statistical procedures
Statistical analysis of the numerical data from all the experiments was performed using anova, followed by Student–Newman–Keuls test. Optical density (OD) of Western blots was assessed by NIH image software for each individual blot and a respective loading control. The main OD values were divided by loading control OD and normalized to OD values in untreated cells. The latter were assigned an arbitrary value of ‘1’. This was done for each independent experiment and the mean was calculated.
Results
Tianeptine protects from gp120-induced apoptosis
To evaluate potential protective effects of tianeptine against HIV-induced neurodegeneration, we used Lipari human astroglial cells as an in vitro model (Visalli et al., 2007). Gp120 induced a dose-dependent reduction in cell viability within 24 h (Figure S1A,B) and this reached 45% after 72 h. Importantly, the incubation with tianeptine significantly reduced the percentage of trypan blue positive astrocytes in a dose-dependent manner (Figure 1A), but had no statistically significant effect on control cells. Cell death caused by gp120 was mainly due to apoptosis, as demonstrated by Annexin V staining (Figure 1B,C) and confirmed by TUNEL assay (Figure S1A,B). In fact, 7-AAD positive, but Annexin V negative cells were in the minority of dying cell population even at 48 h post-gp120 treatment. Tianeptine protected the astrocytes from apoptosis induced by gp120 in a dose-dependent manner (Figure 1B,C and Figure S1B). Again, no statistically significant change in basal apoptosis was induced by tianeptine alone (Figure 1B). Gp120-induced apoptosis involves caspase-3 activation in lymphocytes and neuronal cells (Garden et al., 2002; Perfettini et al., 2005). Therefore, we determined whether caspase-3 is also activated in astroglial cells in response to gp120. After gp120 treatment in the presence or absence of tianeptine, the cells were stained with FITC-z-VAD-fmk, a fluorescent version of irreversible caspase-3 inhibitor. Gp120 significantly activated caspase-3 compared with control cells (Figure 1D,E) in a dose-dependent manner (Figure 1D). The actual percentage of apoptotic cells in response to gp120 was dependent on cell density at the beginning of the treatment (Figure S1C,D). The protective effect of tianeptine was also dependent on cell density and the drug exerted maximal protection at 5 µM if applied for 48 h on cells growing at high density (Figure S1D).
Figure 1.
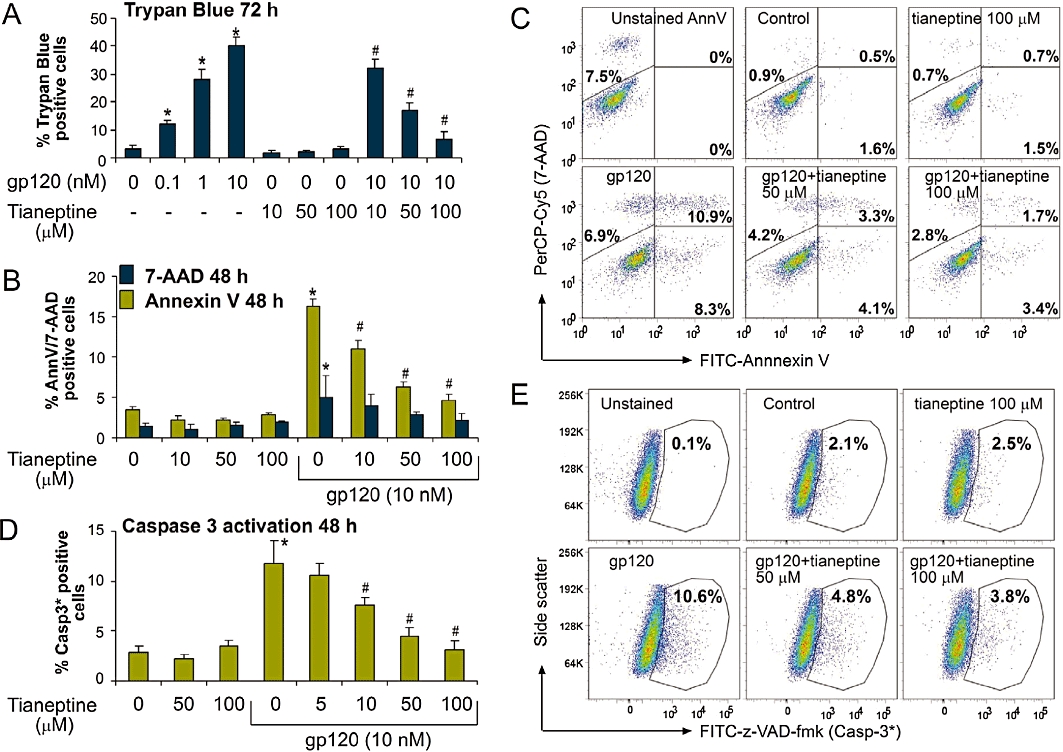
Tianeptine prevents gp120-induced apoptotic cell death in cultured astroglial cells. (A) Lipari astroglial cells were treated with gp120 for 72 h. Tianeptine was added 1 h before the addition of gp120. Cell mortality was assessed by % trypan blue positive cell counts after enzymatic cell detachment by trypsin. Data represent the mean of eight independent experiments ± SEM. The * indicates a statistically significant difference at P < 0.05 for gp120-treated astrocytes versus control; # indicates a statistically significant change at P < 0.05 for gp120 plus tianeptine versus gp120 only-treated astrocytes. (B) Incubation of Lipari astroglial cells with gp120 (10 nM) for 48 h induced mainly apoptotic cell death, as assessed by FITC-Annexin V/7-AAD double labelling followed by FACS bi-parametric analysis. Tianeptine (10, 50, 100 µM) prevented the effect of gp120. Cells (0.8 × 104 cm−2) were seeded 24 h before the treatment. Annexin V columns show the percentage of total Annexin V positive cells, early (Annexin V positive 7-AAD negative) and -late (double stained) apoptotic cells; 7-AAD only columns show the percentage of Annexin V negative, 7-AAD positive cells which correspond mainly to necrotic cells. Data represent the mean ± SEM of three independent experiments. *, # − see (A). (C) Representative dot plots of FITC fluorescence versus 7-AAD fluorescence for the experiment shown in (B). Unstained AnnV – cells stained with 7-AAD, but not with FITC-Annexin V. (D) Incubation of U373 astroglial cells with gp120 for 48 h leads to caspase-3 activation as shown by the increased binding to FITC-conjugated z-VAD-fmk. Tianeptine antagonized the caspase-3 cleavage in response to gp120. U373 astroglial cells were treated and untreated with tianeptine at different concentrations and then stimulated or not with gp120 (10 nM). 48 h later the cells were incubated with FITC-z-VAD-fmk and processed for FACS analysis, followed by FlowJo analysis of FITC-positive gate. The experiment was performed four times in triplicate. *, # − statistical analysis was performed as in (A). (E) Representative dot plots of FITC fluorescence versus side-scatter for the experiment shown in (D).
Tianeptine modulates GS levels and activity
We have previously shown that glutamine synthase protein levels are down-regulated in gp120-treated astroglial cells and antioxidant N-acetylcysteine treatment was able to partially restore normal GS levels (Visalli et al., 2007). We determined whether the protective action of tianeptine also correlates with an increase in GS levels and/or activity. To this end, we pretreated the astroglial cells with different concentrations of tianeptine (10–100 µM) before the addition of gp120 and 24 h later determined the glutamine concentration in the medium as an indirect measure of GS activity and also evaluated GS protein levels in cell lysates in the same experiment. As expected, gp120 markedly reduced GS protein expression compared with untreated cells (Figure 2B). This correlated with a reduction in glutamine concentration by 75%, suggesting a significant decrease in GS activity in these cells (Figure 2A). Importantly, the presence of tianeptine restored both GS parameters in a dose-dependent manner (Figure 2A,B).
Figure 2.
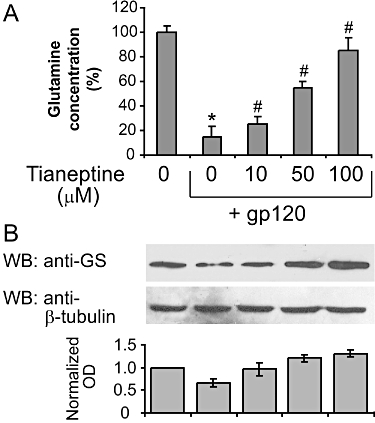
Tianeptine enhances glutamine synthase (GS) levels and activity in astroglial cells. (A) The decrease in glutamine concentration in the supernatant of astroglial cells incubated with gp120 is reversed by tianeptine. Treatment of astroglial cells with gp120 (10 nM) for 24 h produced a reduction in glutamine formation compared with the untreated control. Tianeptine (10, 50, 100 µM) reversed this effect. Data represent the mean ± SEM of five independent experiments. *P < 0.05 when compared with control; #P < 0.05 as compared to gp120 alone-treated cells. (B) The effect of gp120 on GS expression levels in astroglial cells. Incubation of astroglial cells with gp 120 (10 nM) for 24 h dose dependently reduced the GS expression. Pretreatment for 2 h with tianeptine (10, 50, 100 µM) antagonized this effect. Blots are representative of four independent experiments. Normalized optical density of GS signal from four experiments is shown next. Data represent the mean ± SEM.
Tianeptine modulation of NO synthesis and iNOS and cNOS levels
We and others have demonstrated that treatments with gp120 and other pro-inflammatory stimuli such as LPS and INF-γ, result in a rapid and transient reduction in NO levels, reflecting inhibition of cNOS activity (Colasanti et al., 1997). This precedes a sustained up-regulation of inducible NOS and subsequent excessive increase in NO (Mollace et al., 1993). Therefore, we measured the nitrate levels 30 min after the treatment with gp120 to detect the transient NO decrease in the presence and absence of tianeptine. Tianeptine 100 µM prevented the reduction in nitrate concentration elicted by gp120 (Figure 3A). This correlated with changes in the expression of the neuronal isoform of cNOS (nNOS) measured 24 h after the addition of gp120 or gp120 plus tianeptine in cell lysates from the same experiment (Figure 3B). Moreover, tianeptine prevented the induction of iNOS observed 24 h later upon the addition of gp120 in the same experiment (Figure 3D). The changes in iNOS and nNOS levels induced by gp120 and tianeptine were reflected by nitrate levels in the cell culture supernatant assessed 24 h after the addition of both factors (Figure 3C). Indeed, the induction of iNOS by gp120 led to a strong increase in nitrates concentration (sevenfold), while co-treatment with tianeptine suppressed NO production in a dose-dependent fashion.
Figure 3.
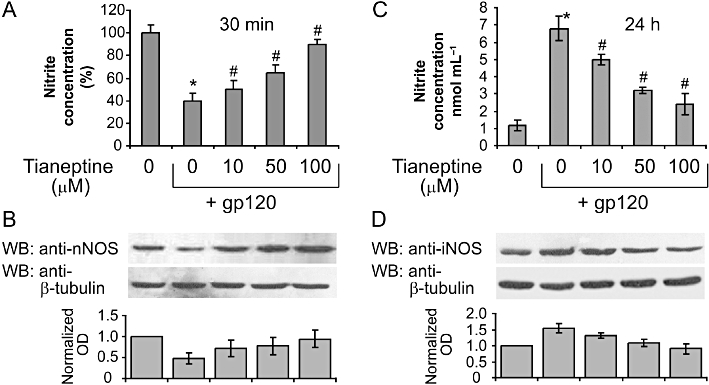
Tianeptine effect on nitric oxide and nitric oxide synthases. (A) Tianeptine prevents the transient decrease in nitrite levels in the supernatant of astroglial cells incubated with gp120. Treatment of astroglial cells with gp120 (10 nM) for 30 min produced an initial decrease of nitrite levels compared with untreated controls. Tianeptine (10, 50, 100 µM) reversed this effect dose dependently. Data represent the mean ± SEM of five independent experiments normalized to and expressed as percentage of nitrite concentration in untreated cells. *P < 0.05 when compared with control; #P < 0.05 tianeptine-treated versus gp 120 only treated cells. (B) The effect of gp120 on nNOS levels in human cultured astroglial cells. Incubation of cells with gp 120 (10 nM) for 24 h dose dependently reduced the nNOS expression as measured by Western blotting analysis. Tianeptine (10, 50, 100 µM), antagonized this effect dose dependently. Blots are representative of four independent experiments. Normalized optical density of nNOS signal from four experiments is shown next. Data represent the mean ± SEM. (C) Tianeptine prevents a later increase in nitrite levels in the supernatant of astroglial cells incubated with gp120 for 24 h. The experiment was performed as in (A) but the nitrate levels were assessed after 24 h. Data represent the mean ± SEM of three independent experiments. *,# − see (A). (D) Changes in protein levels of iNOS in a similar experiment to that in (B). Blots are representative of three independent experiments. Normalized optical density of nNOS signal from three experiments is shown below. Data represent the mean ± SEM.
Tianeptine prevents activation of NF-κB by controlling IκB-α levels
The main signalling route responsible for the induction of iNOS is the classical NF-κB pathway. Gp120 was shown to activate NF-κB in lymphoblastic and other CD4 expressing cell lines (Bossis et al., 2002). We evaluated the activation of NF-κB by monitoring changes in IκB-α levels and NF-κB promoter activity in astroglial cells. First we checked the effect of gp120 on IκB-α degradation. As shown in Figure 4A, gp120 induced a significant drop in IkB-α levels, detected 30, 40 and 60 min after its addition. Next we selected the 40 min treatment with gp120 as the optimal time to evaluate possible effects of tianeptine on IkB-α degradation in astroglial cells. Tianeptine pretreatment for 1 h counteracted the gp120-induced decrease in IκB-α levels in a dose-dependent manner (Figure 4C); the effect induced by 50 µM tianeptine correlated with a significant reduction in NF-κB promoter activity, as determined by luciferase reporter assay 5 h after gp120 stimulation (Figure 4D). Interestingly, tianeptine at doses from 5 up 100 µM increased the basal levels of IκB-α (Figure 4B). Accordingly, we observed reduced NF-κB luciferase activity in cells pretreated with tianeptine alone compared with control cells (Figure 4D).
Figure 4.
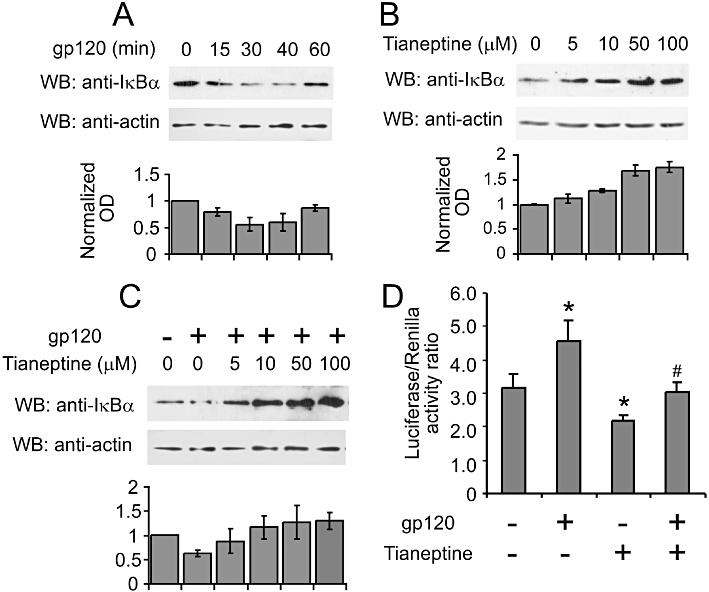
Tianeptine interferes with NF-κB signalling by stabilizing IκB-α in astroglial cells. (A) Time-course analysis of IκB-α protein levels in response to gp120 stimulation. The astroglial cells (seeded 1.5 × 104 cm−2 cells 24 h before the treatments) were stimulated with 10 nM gp120 and lysed in RIPA buffer at indicated time points. Lysates were processed and 5 µg of total protein lysates were loaded on the 10% SDS-PAGE gel and subsequently analysed by Western blotting. The blot is representative of three independent experiments. The graph represents the normalized mean OD of IκB-α signal divided by mean OD of actin signal from three independent blots ± SEM. (B) Tianeptine stabilizes IκB-α in the absence of gp120 stimulation. The cells seeded as in (A) were treated for 1 h 40 min with different concentrations of tianeptine and processed as in (A) for Western blotting. The graph represents the normalized OD of IκB-α signal from analysed as in (A). (C) Tianeptine inhibits the degradation of IkBa induced by gp120. The astroglial cells were seeded as in (A) and stimulated with gp120 for 35 min. Tianeptine at different doses was added 1 h before the administration of gp120. The cells were processed for Western blotting as in (A). The graph shows the normalized OD of IκB-α signal analysed as in (A) from 3 independent experiments. (D) NF-κB transcriptional activity is suppressed by tianeptine. The astroglial cells were transfected with pNfkB-Luc and pRL reporter plasmids. Twenty hours post-transfection, the cells were stimulated with 10 nM gp120 and/or tianeptine (50 µM) for 5 h or left untreated. Each treatment was performed six times. Luciferase activity and internal control renilla activity are the mean of six experimental points. Data represent the mean ± SEM of a representative experiment, which was performed three times. *P < 0.05 when compared with control; #P < 0.05 tianeptine and gp120 versus gp120 alone-treated cells.
These data indicate that tianeptine protects the astrocytes from apoptotic cell death by interfering with the early events triggered by gp120, such as degradation of IκB-α followed by NF-κB activation.
Discussion and conclusions
GS is the fundamental enzyme for astroglial and neuronal protection as it catabolizes excessive amounts of glutamate released by damaged cells (Oliver et al., 1990; Tuor et al., 1995, 1996; Bertorelli et al., 1998). The reactive increase of GS activity occurs after brain injury and it is protective in gp120-related astrocytic dysfunction (Muscoli et al., 2005). Therefore, the increased GS activity induced by tianeptine (Figure 2A), which counteracts the down-regulation of GS induced by incubation of astroglial cells with gp120, might be able to protect astrocytes from HIV-related dysfunction. However, this increase in GS activity is likely to be driven, at least in part, by NO release from the neuronal isoform of constitutive NO synthase (nNOS). Indeed, the dose-response curves for the effects of tianeptine on GS activity and nitrate levels were perfectly superimposable, indicating that these effects cannot be separated in terms of neuroprotection and are directly dependent on each other (Figure 5A). This suggests that nanomolar concentrations of NO directly regulate GS, in accordance with data from our and other groups showing that constitutive release of NO by cNOS is necessary to sustain GS activity in the adaptive response of astrocytes to inflammatory stimuli (Minana et al., 1997). In particular, we have previously shown that NMDA-related, constitutive release of NO correlates with enhanced GS expression in astroglial cells. On the other hand, the activation of iNOS by LPS plus IFN-γ has been shown to reduce NMDA-related elevation of GS (Muscoli et al., 2005). This latter effect most likely occurs because the overproduction of NO as a later consequence of exposure of astrocytes to gp120 impairs GS activity and stability, possibly via nitration and nitrosylation as shown by others (Gorg et al., 2007). Thus, the return of nNOS towards basal levels may represent a crucial mechanism activated by tianeptine, which could be involved in its anti-apoptotic effect on gp120-induced apoptosis (Figure 5B).
Figure 5.
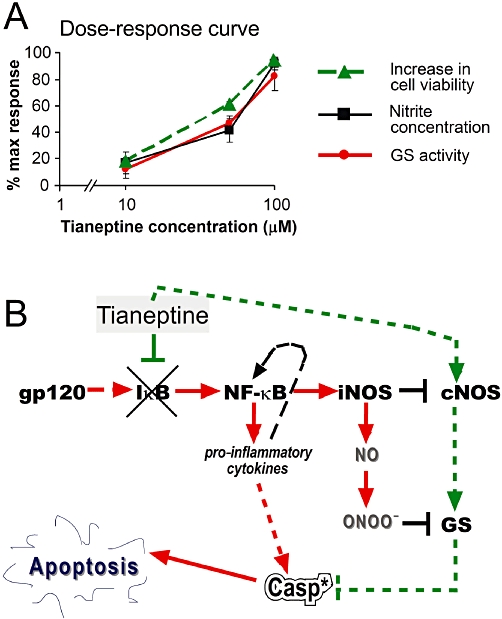
(A) Dose-response curve for effects of tianeptine on cell viability and enzyme activities counteracting pro-apoptotic effects of gp120. The response was calculated according to Figure 1 as the increase in cell viability (i.e. 100% – % trypan blue positive cells) compared with viability in the presence of 10 nM gp120. For glutamine synthase (GS) and nNOS activity (measured indirectly by nitrite concentration), the data from Figures 2A and 3A were recalculated, assuming that the gp120-induced suppressed activity corresponds to 0, while 100% activity corresponds to control untreated cells. Note that GS activity and nitrite levels are superimposable. (B) The schematic representation of molecular mechanisms induced by tianeptine. The primary effect of tianeptine may involve the block of gp120-induced cNOS down-regulation and IκB-α degradation. This may prevent the induction of iNOS and decrease in cNOS levels, thus stabilizing low physiological NO levels, which finally increase GS activity. High GS activity is fundamental for protection from apoptosis. Inhibition of NF-κB by tianeptine may attenuate other pro-inflammatory responses to gp120 comprising an increased production of pro-inflammatory cytokines. *, activated caspase-3.
Evidence exists that NO released under basal conditions by cNOS maintains iNOS in a non-activated state through the inhibition of the NF-κB signalling, which in turn regulates the iNOS by transcriptional mechanisms (Colasanti and Suzuki, 2000). NF-κB represents one of the most important signalling pathways that mediate the expression of iNOS following the addition of a variety of extracellular mediators, including endotoxin and inflammatory cytokines (Bonaiuto et al., 1997; Chao et al., 1997; Massa and Wu, 1998; Hartlage-Rubsamen et al., 1999). When low concentrations of endogenous as well as exogenous NO are released, NF-κB is kept in an inactive state (Togashi et al., 1997). Many factors may contribute to NO-related inhibition of NF-κB, including a direct effect of NO on the binding of NF-κB to its promoter response element without affecting the activation and translocation of NF-κB (Matthews et al., 1996; DelaTorre et al., 1997). NO has been shown to inhibit NF-κB DNA binding through S-nitrosylation of the Cys 62 residue of p50 subunit (delaTorre et al., 1998). Moreover, NO and NO donors have been shown to inhibit NF-κB more upstream, via the stabilization of its inhibitor IkB-α, without affecting the mRNA expression of NF-κB subunits p65 or p50 (Peng et al., 1995). Similarly, in our hands, tianeptine prevented an early down-regulation of IκB-α (30 min) induced by gp120 (Figure 4A,C), needed for the activation of the classical NF-κB pathway and iNOS induction. Interestingly, our data show that tianeptine stabilizes IκB-α even in the absence of gp120 (Figure 4B), indicating that astroglial cells in culture have elevated levels of basal NF-κB signalling, which can be further increased by gp120, or reduced by tianeptine (Figure 4D). We do not know if the tianeptine-dependent block of IκB-α degradation is a result of its early positive effect on nNOS levels (Figure 3A,B) and nanomolar levels of NO (Peng et al., 1995) or induced by other unknown mechanisms. However, it is likely that the inhibitory effect of tianeptine on iNOS (Figure 3C,D) is mediated by suppression of NF-κB activation.
These novel findings suggest a mechanism for the anti-inflammatory effects of tianeptine reported previously; tianeptine was shown to attenuate the pro-inflammatory effects of IL-1β in the CNS (Plaisant et al., 2003) and LPS-induced expression of TNF-α in the spleen and TNF-α plasma levels (Castanon et al., 2004). Moreover, chronic tianeptine treatment altered the balance between pro- (IL-1β, TNF-α) and anti-inflammatory cytokines (IL-10) in the hypothalamus following LPS injection. As cytokine expression and inflammation are regulated by NF-κB, it is likely that the inhibitory action of tianeptine on NF-κB signalling observed by us may represent a common mechanism for the anti-inflammatory and anti-apoptotic effects of this drug.
Thus, while the augmentation of GS activity has been claimed to play a pivotal role in the therapeutic effects of tianeptine (Roegel and Eftekhari, 2009), this action is only one of many potential downstream effects of tianeptine, most likely secondary to its effects on cNOS and iNOS, IκB and NF-κB as depicted in Figure 5B.
In conclusion, our data show that tianeptine, a novel antidepressant drug, attenuates the pro-apoptotic effect of the HIV-coating gp120 neurotoxin in human astrocytes. Tianeptine restores GS activity and expression in astroglial cells following incubation with gp120 by regulating the balance between constitutive and inducible release of NO by astroglial cells. This effect correlated with the inhibition of NF-κB transcription factor at the level of IκB-α stabilization.
As shown in our model of molecular circuits activated by gp120 and conteracted by tianeptine (Figure 5B), an initial decrease in NO is necessary for NF-κB triggering and subsequent iNOS induction. In contrast, the inhibition of this decrease will prevent iNOS induction. Indeed, when tianeptine is added to gp120-treated astrocytes, nNOS is re-activated and iNOS expression is attenuated (Figure 3), thus indicating that tianeptine may allow the NO generating system to work under a ‘non-inflamed’ state of efficiency. This model suggests a mechanism for the previously described anti-inflammatory effects of tianeptine (Plaisant et al., 2003; Castanon et al., 2004) and indicate its potential as a drug for the treatment of neurodegenerative pro-inflammatory changes in the CNS that accompany HIV infection in a substantial group of AIDS patients.
Acknowledgments
We thank Prof Stefano Aquaro and Dr Michela Pollicita, University Tor Vergata, Rome, Italy for the kind gift of U373 astroglial cells. We are grateful to Dr Cinzia Raso for the help and technical support. We thank Alessia Maretta and Antonino D'Agostino for their technical assistance. This work was supported by ARPACal (Regional Environmental Agency of Calabria, Italy) science funding.
Glossary
Abbreviations
- Abs
antibodies
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CMV
cytomegalovirus
- cNOS
constitutive nitric oxide synthase
- FITC
fluorescein isothiocyanate
- GLT1
glutamate transporter 1
- Gp120
glycoprotein 120
- GS
glutamine synthase
- HIV
human immunodeficiency virus
- IκB
inhibitor of nuclear factor κB
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κB
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- z-VAD-fmk
benzyloxycarbonyl-Val-Ala-Asp fluoromethyl ketone
Conflict of interest
None
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Caspase-3 activation by gp120 and the antiapoptotic effect of tianeptine in astroglial cells is cell density dependent. (A) Incubation of Lipari astroglial cells with gp120 (0.1, 1 and 10 nM) for 24 h induced apoptotic cell death, as assessed by TUNEL assay followed by FACS analysis. Cells (0.8 × 104 cm−2) were seeded 24 h before the treatment. Data represent the mean ± SEM of three independent experiments. The * indicates a statistically significant difference at P < 0.05 for gp120-treated astrocytes versus control; # indicates a statistically significant change at P < 0.05 for gp120 plus tianeptine versus gp120 only-treated astrocytes. (B) Tianeptine (10, 50, 100 μM) in a dose-dependent manner prevented the effect of gp120. The cells were seeded as above and the drug was added 1 h before the addition of gp120. *, # – see (A). (C) U373 astroglial cells were seeded at the density 0.5 × 104 cm−2. Next day, the cells were treated and untreated with tianeptine at different concentrations and then stimulated or not with gp120 (10 nM). Then 48 h later the cells were incubated with FITC-z-VAD-fmk and processed for FACS analysis, followed by FlowJo analysis of FITC-positive gate. The experiment was performed three times in triplicate. The graph shows the means of a representative experiment and SEM. (D) The cells were seeded at 5.0 × 104 cells cm−2. Next day, the cells were treated as in (A) followed by the analysis 48 h post-treatment as in (A). The graph shows one of three experiments performed in triplicate that yielded similar results and SEM. Note that the dense cells were much more resistant to apoptosis induced by gp120 and lower concentrations of tianeptine fully protected these cells from caspase-3 activation. In A, B, C, D, statistical analysis was performed by anova.
Flow cytometric analysis of TUNEL positive cells.
Astroglial apoptotic nuclei produced after incubation of astrocytes with gp120 were assessed by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labelling (TUNEL) of DNA strand breaks by using a commercial kit from Promega (cat. #G3250). Briefly, astroglial cells after required treatments were trypsinized, gently detached from plastic and then processed according to manufacturer's instructions as cells in suspension. Propidium iodide-stained hypoploid and TUNEL positive cells were analysed by FACS Canto II Flow Cytometer with 530 and 585 nm channels.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–44. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Bonaiuto C, McDonald PP, Rossi F, Cassatella MA. Activation of nuclear factor-kappa B by beta-amyloid peptides and interferon-gamma in murine microglia. J Neuroimmunol. 1997;77:51–56. doi: 10.1016/s0165-5728(97)00054-4. [DOI] [PubMed] [Google Scholar]
- Bossis G, Salinas S, Cartier C, Devaux C, Briant L. NF-kappaB activation upon interaction of HIV-1 envelope glycoproteins with cell surface CD4 involves IkappaB kinases. FEBS Lett. 2002;516:257–264. doi: 10.1016/s0014-5793(02)02566-8. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, et al. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Castanon N, Bluthe RM, Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology (Berl) 2001;154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- Castanon N, Konsman JP, Medina C, Chauvet N, Dantzer R. Chronic treatment with the antidepressant tianeptine attenuates lipopolysaccharide-induced Fos expression in the rat paraventricular nucleus and HPA axis activation. Psychoneuroendocrinology. 2003;28:19–34. doi: 10.1016/s0306-4530(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Castanon N, Medina C, Mormede C, Dantzer R. Chronic administration of tianeptine balances lipopolysaccharide-induced expression of cytokines in the spleen and hypothalamus of rats. Psychoneuroendocrinology. 2004;29:778–790. doi: 10.1016/S0306-4530(03)00142-2. [DOI] [PubMed] [Google Scholar]
- Chao CC, Lokensgard JR, Sheng WS, Hu S, Peterson PK. IL-1-induced iNOS expression in human astrocytes via NF-kappa B. Neuroreport. 1997;8:3163–3166. doi: 10.1097/00001756-199709290-00031. [DOI] [PubMed] [Google Scholar]
- Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2000;21:249–252. doi: 10.1016/s0165-6147(00)01499-1. [DOI] [PubMed] [Google Scholar]
- Colasanti M, Cavalieri E, Persichini T, Mollace V, Mariotto S, Suzuki H, et al. Bacterial lipopolysaccharide plus interferon-gamma elicit a very fast inhibition of a Ca2+-dependent nitric-oxide synthase activity in human astrocytoma cells. J Biol Chem. 1997;272:7582–7585. doi: 10.1074/jbc.272.12.7582. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- delaTorre A, Schroeder RA, Bartlett ST, Kuo PC. Differential effects of nitric oxide-mediated S-nitrosylation on p50 and c-jun DNA binding. Surgery. 1998;124:137–141. discussion 141–2. [PubMed] [Google Scholar]
- DelaTorre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B p50 DNA binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238:703–706. doi: 10.1006/bbrc.1997.7279. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D'Emilia DM, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- Hartlage-Rubsamen M, Lemke R, Schliebs R. Interleukin-1beta, inducible nitric oxide synthase, and nuclear factor-kappaB are induced in morphologically distinct microglia after rat hippocampal lipopolysaccharide/interferon-gamma injection. J Neurosci Res. 1999;57:388–398. [PubMed] [Google Scholar]
- Huang TL, O'Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–1442. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Voaden MJ, Marshall J. Glutamate metabolism in the frog retina. Nature. 1974;252:50–52. doi: 10.1038/252050a0. [DOI] [PubMed] [Google Scholar]
- Kole MH, Swan L, Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10:525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- Massa PT, Wu C. Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res. 1998;18:499–507. doi: 10.1089/jir.1998.18.499. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucl Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minana MD, Kosenko E, Marcaida G, Hermenegildo C, Montoliu C, Grisolia S, et al. Modulation of glutamine synthesis in cultured astrocytes by nitric oxide. Cell Mol Neurobiol. 1997;17:433–445. doi: 10.1023/a:1026339428059. [DOI] [PubMed] [Google Scholar]
- Mollace V, Colasanti M, Persichini T, Bagetta G, Lauro GM, Nistico G. HIV gp120 glycoprotein stimulates the inducible isoform of no synthase in human cultured astrocytoma cells. Biochem Biophys Res Commun. 1993;194:439–445. doi: 10.1006/bbrc.1993.1839. [DOI] [PubMed] [Google Scholar]
- Mollace V, Salvemini D, Riley DP, Muscoli C, Iannone M, Granato T, et al. The contribution of oxidative stress in apoptosis of human-cultured astroglial cells induced by supernatants of HIV-1-infected macrophages. J Leukoc Biol. 2002;71:65–72. [PubMed] [Google Scholar]
- Muscoli C, Visalli V, Colica C, Nistico R, Palma E, Costa N, et al. The effect of inflammatory stimuli on NMDA-related activation of glutamine synthase in human cultured astroglial cells. Neurosci Lett. 2005;373:184–188. doi: 10.1016/j.neulet.2004.09.079. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, et al. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, et al. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12(Suppl 1):916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- Plaisant F, Dommergues MA, Spedding M, Cecchelli R, Brillault J, Kato G, et al. Neuroprotective properties of tianeptine: interactions with cytokines. Neuropharmacology. 2003;44:801–809. doi: 10.1016/s0028-3908(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Hendry RM, Reznikov LR, Piroli GG, Wood GE, McEwen BS, et al. Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur J Pharmacol. 2007;565:68–75. doi: 10.1016/j.ejphar.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Roegel JC, Eftekhari P. Novel uses for drugs targeting glutamine synthetase. 2009. In US Patent USA: 0209474 A1.
- Spedding M, Lestage P. [Synaptic plasticity and neuropathology: new approaches in drug discovery] Med Sci (Paris) 2005;21:104–109. doi: 10.1051/medsci/2005211104. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, et al. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci. 2007;26:3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, et al. Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci USA. 1997;94:2676–2680. doi: 10.1073/pnas.94.6.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuor UI, Chumas PD, Del Bigio MR. Prevention of hypoxic-ischemic damage with dexamethasone is dependent on age and not influenced by fasting. Exp Neurol. 1995;132:116–122. doi: 10.1016/0014-4886(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Del Bigio MR, Chumas PD. Brain damage due to cerebral hypoxia/ischemia in the neonate: pathology and pharmacological modification. Cerebrovasc Brain Metab Rev. 1996;8:159–193. [PubMed] [Google Scholar]
- Visalli V, Muscoli C, Sacco I, Sculco F, Palma E, Costa N, et al. N-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunction. BMC Neurosci. 2007;8:106. doi: 10.1186/1471-2202-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


