Abstract
Thrombospondin 1 (TSP1), an oligomeric matrix protein, is known for its antiangiogenic activity. Recently, TSP1 has been shown to regulate synaptogenesis in the developing brain. In this study, we examine another role of TSP1 in the CNS, namely, in proliferation and differentiation of neural progenitor cells (NPCs). We found that adult mice deficient in TSP1 exhibit reduced proliferation of NPCs in vivo [13,330±826 vs. 4914±455 (mean±se wt vs. TSP1−/−); P<0.001, Student’s t test] and impaired neuronal differentiation (1382±83 vs. 879±79; P<0.001). In vitro, NPC obtained from adult TSP1−/− mice display decreased proliferation in BrdU assay (48±8 vs. 24±3.5%; P<0.01) and decreased neuronal fate commitment (8±0.85 vs. 4.6±0.5%; P<0.05) in contrast to wild-type NPCs. Both proliferation and neuronal differentiation deficits are remediable in vitro by exogenous TSP1. Notably, conditioned medium from TSP1−/− astrocytes, unlike that from control astrocytes, fails to promote neurogenesis in wild-type NPCs, suggesting that TSP1 is one of the key molecules responsible for astrocyte-induced neurogenesis. Our data demonstrate that TSP1 is a critical participant in maintenance of the adult NPC pool and in neuronal differentiation.—Lu, Z., Kipnis, J. Thrombospondin 1—a key astrocyte-derived neurogenic factor.
Keywords: neural progenitor cells, adult neurogenesis, oligodendrogenesis, extracellular matrix proteins
The adult mammalian brain contains neural progenitor cells (NPCs) that are responsible for the production of new neurons and glia (astrocytes and oligodendrocytes) throughout the life of an organism. While myriad brain areas have been shown at varying developmental stages to contain NPCs, there exist only two active neurogenic zones (niches) in the adult brain; these are the subventricular zone (SVZ) of the lateral ventricles and the subgranular layer (SGL) of the hippocampal dentate gyrus (1,2,3). These zones may be viewed as developmental “islands” in the adult brain.
Extrinsic factors, including cognitive tasks, physiological and psychological stresses, and immune factors, robustly affect NPC proliferation and differentiation (4,5,6,7). Moreover, to a great extent, the input from adjacent neurons and astrocytes determines the fate of NPCs or newly differentiated neural cells. Astrocytes are essential constituents of the neurogenic niches and produce soluble and membrane-bound factors to promote neuronal differentiation from NPCs (8). In fact, astrocytes not only support neurogenesis, but also promote synaptogenesis in the developing brain. Recently, extracellular matrix (ECM) proteins, thrombospondins (TSPs) 1 and 2 were discovered to be the essential astrocyte-derived synaptogenesis-promoting factors (9).
TSPs are large oligomeric ECM proteins that mediate cell-cell and cell-matrix interactions by binding with other ECM proteins and with an array of membrane receptors and cytokines. Studies have shown that TSPs participate in cell attachment, proliferation, and differentiation, as well as in apoptosis and in inhibition of angiogenesis (10,11,12,13). It was recently suggested that TSPs are active players in the developing central nervous system (CNS), and experimental evidence collected thus far has borne out this hypothesis. Both TSP1 and TSP2 are critical for the support of astrocyte-induced synaptogenesis (9); astrocytes from TSP1/2−/− mice cannot support synaptogenesis in retinal ganglion cells (RGCs) in vitro. The addition of exogenous purified TSP1, however, to the growing culture of RGCs recapitulates the effect of astrocytes or of astrocyte-conditioned media on synaptogenesis. TSP1 has also been shown to promote attachment of neurons and outgrowth of neurites in culture (14,15,16). Recently, TSP1 was shown to be a crucial factor affecting migration of neuronal precursor cells [doublecortin (DCX)-positive cells] in vivo in early postnatal [postnatal day 17 (p17)] brain (17).
Here, we propose that TSP1, which is expressed in adult brain, plays a key role in NPC proliferation and neuronal differentiation in vivo and in vitro.
MATERIALS AND METHODS
Animals
TSP1−/− and TSP2−/− mice (on a B6129SF1/J background) and their relative B6129SF1/J wild-type (WT) controls were bred in the animal facilities at the University of Virginia. The original breeding pairs were kindly provided by Dr. Paul Bornstein (University of Washington, Seattle, WA, USA). The animals were housed in light- and temperature-controlled rooms and age-matched in each experiment. Both strains were kept in identical housing conditions, and mice at ages 8–12 wk were used throughout the study (young adult mice). All experiments were conducted under the supervision of the University of Virginia Animal Care Advisory Committee in accordance with an approved animal use protocol that adhered to practices outlined in the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Neurosphere culture
Neurospheres were generated from the SVZ of young adult mice, as described previously (18) and cultured under clonal conditions, according to published procedures (19). Briefly, for each culture, the entire SVZ region was dissected from sagittally cut brains and incubated in DMEM containing 20 U/ml papain (Worthington, Lakewood, NJ, USA), 1 mM N-acetyl-l-cysteine (NAC; Sigma, St. Louis, MO, USA) and 2 U/ml RQ1 DNase (Promega, Madison, WI, USA) for 1 h at 37°C. Dissociated cells were plated in 24-well plates at a density of 2.6 × 103 cells/cm2 (5×103 cells in 0.5 ml/well) in high-glucose DMEM containing N2 (Invitrogen, Carlsbad, CA, USA), B-27 (StemCell Technologies, Vancouver, BC, Canada), 1 mM NAC, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 ng/ml EGF, and 20 ng/ml bFGF (both growth factors recombinant human from Invitrogen). The total number of neurospheres at passage 0 (P0) was determined at 7 days in vitro (7 DIV) by counting all neurospheres generated from each mouse brain. In subsequent passages, the total number of neurospheres was determined by counting all neurospheres generated in a 24-well dish seeded at 5 × 103 cells/well and extrapolating for the total number of dissociated cells. Self-renewal was determined as the number of secondary neurospheres/number of seeded cells × 100 (i.e., how many early precursors capable of generating a “daughter” neurosphere were contained in each “mother” neurosphere). Amplification was calculated as the number of viable cells from dissociated neurospheres/number of seeded cells (i.e., how much the overall number of cells had grown during each cycle). The number of viable cells was determined in samples of SVZ- or neurosphere-dissociated cells by trypan blue exclusion. Neurosphere diameter (>40 μm) was measured in phase-contrast pictures of individual neurospheres using QCapture Pro 6.0 software (QImaging, Surrey, BC, Canada). For the cell differentiation study, neural spheres were dissociated in papain or accutase (Invitrogen) solution. Dissociated single cells were seeded on the coverslips, which were covered with poly-l-lysine. Cells were kept in differentiation medium for either 7 or 14 d, with half of the medium changed every other day.
Flow cytometry
P5 neurospheres were gently dissociated with Accutase (Invitrogen) in NSC culture medium. Cells were then labeled with antibodies to CD133 (conjugated to FITC), CD24 (conjugated to PE) (eBioscience, San Diego, CA, USA), and DRAQ5 (Biostatus, Shepshed, UK). Fluorescence data were collected with a BD FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA) and then analyzed using Flowjo (Tree Star, Ashland, OR, USA).
qRT-PCR
Total mRNA was extracted from either brain tissue or NPCs, followed by cDNA synthesis by RNAqueous-Mico kit (Ambion, Austin, TX, USA). The following primers were used to detect mRNA levels: TSP1, forward CAAGGGCTCAGGGATACTCAGG and reverse AGGTTTTGTCATAGATGGGTCC; TSP2, forward TCGTTGGAGACCAGTGTGACAACA and reverse TGTCATCAGAGTCGCAGGCAT; β-actin, forward CTGTATTCCCCTCCATCGTG and reverse CTGACCCATTCCCACCATCAC.
Bromodeoxyuridine (BrdU) treatment
BrdU (Sigma, St. Louis, MO, USA) was dissolved in 0.9% saline containing 0.007 M NaOH at a concentration of 5 mg/ml. To label proliferating cells in the brain, mice received 2 injections of BrdU (50 mg/kg; i.p.) 2 h apart. For examination of progenitor proliferation, mice were euthanized 24 h after the second injection, whereas for examination of progenitor differentiation, mice were euthanized 7 d after BrdU injection. Mice were perfused with PBS for 3 min and 4% PFA in pH 7.2 PBS for 5 min. Brains were excised and postfixed in 4% PFA for 72 h at 4°C, then in 30% sucrose for at least 2 d. Brains were frozen in 2-methylbutane Chromasolv with dry ice, stored at −80°C until ready for cryostat slicing. Coronal sections (40 μm thick) were obtained. Six sequential slices were placed into each well of a 24-well plate containing 0.02% sodium azide in PBS and stored at 4°C.
To quantify proliferating progenitor cells, we used a semistereological approach, widely used by other labs (4, 20). Every sixth section (40 μm thick) of the brain containing hippocampus or lateral ventricles was selected and immunolabeled with BrdU (proliferation; rat anti-BrdU, 1:500; Abcam, Cambridge, MA, USA), or DCX (differentiation; guinea-pig anti-DCX, 1:2000; Chemicon, Temecula, CA, USA), or Ki-67 (endogenous proliferation marker; rabbit-anti Ki-67, 1:400; Epitomics, Burlingame, CA, USA) antibodies. Total numbers of positive cells in all slices per animal were multiplied by 6 to estimate the number of cells per hippocampus or lateral ventricles.
To detect BrdU incorporation, neurospheres were incubated with 3 μg/ml (10 μM) BrdU for the last 8 h and then immediately fixed in 4% PFA at room temperature for 30 min.
Immunofluorescence
For BrdU labeling on coronal free-floating sections, the sections were permeabilized with 0.5% Triton X-100 in PBS for 20 min, and then treated with 0.3% H2O2 to block endogenous peroxidases, denatured in 2 N HCl for 15 min, and rinsed twice in 0.1 M (pH 8.5) borate buffer. Sections were placed in 10% normal goat serum in PBS for 1 h, then incubated with primary antibody rat-anti BrdU (1:500; Abcam) overnight at 4°C. For DCX and Ki-67 labeling, brain slices were permeabilized with 0.5% Triton X-100 in PBS for 1 h. Following blocking, the slices were incubated with guinea pig anti-DCX (1:2000; Chemicon) or rabbit-anti Ki-67 (1:400; Epitomics) antibodies, and fluorescent dye conjugated secondary antibodies were used to label the cells.
For BrdU labeling in neurospheres, fixed P1 neurospheres were permeabilized with 0.5% Triton X-100 in PBS for 15 min and then incubated in 2 N HCl at 37°C for 15 min, followed by 3 washes in borate buffer (0.1 M). The neurospheres were then blocked with 10% chicken serum and 0.1% Triton X-100 in PBS for 1 h at room temperature. Neurospheres were then incubated at 4°C for 24 h with rat anti-BrdU antibody and mouse anti-Nestin antibody (1:200; both from Abcam). After 3 washes, Alexa Fluor 488 chicken anti-rat and Alexa Fluor 594 chicken anti-mouse (1:500, Invitrogen) were applied to incubate with specimen at room temperature for 1 h. Confocal Z-stack images were taken, and cell numbers (Nestin+ and BrdU+) were counted using ImageJ software (NIH, Bethesda, MD, USA). The percentage of BrdU+ cells in a sphere was calculated as total BrdU+ cells divided by total Nestin+ cells.
TUNEL labeling
In situ cell death detection kit (cat. no. 11684795001; Roche, Mannheim, Germany) was applied to detect cell apoptosis in the primary neurospheres, according to the manufacturer’s instructions.
NPC differentiation
Dissociated NPCs were plated at 8 × 103 cells/cm2 on polylysine-coated coverslips and maintained in the differentiation medium (high-glucose DMEM containing N2, B-27, 1 mM NAC, 1 mM sodium pyruvate, 2 mM l-glutamine, and 1% FBS) for 7 d. Half of the medium was replaced with fresh medium every other day. For immunofluorescent staining, the following primary antibodies (all at 1:400 dilution) were used on 4% paraformaldehyde-fixed cells: mouse or rabbit anti-β-tubulin isotype III (Sigma), rabbit anti-NG2 (Millipore, Billerica, MA, USA), mouse anti-RIP, and chicken anti-GFAP (Abcam). Fluorescently labeled cells were visualized and imaged at ×40 and ×100 on a confocal microscope (Zeiss LSM510; Carl Zeiss, Oberkochen, Germany). Positive cells were quantified in ≥20 fields systematically across the coverslips from 4 independent experiments of parallel cultures. Statistical analyses were performed using the 2-tailed Student’s t test.
Purified TSP1 protein and astrocyte-conditioned medium (ACM) rescue assay
Purified TSP1 was obtained from Biodesign (Saco, ME, USA). Every 12 h, 2 μg/ml of purified TSP1 was applied to incubate with NPCs. ACM was collected from cultured WT or TSP1−/− astrocytes after 48 h incubation and was concentrated to 5× using Centriprep (Millipore). ACM diluted in medium as 1× ratio was replaced every day.
RESULTS
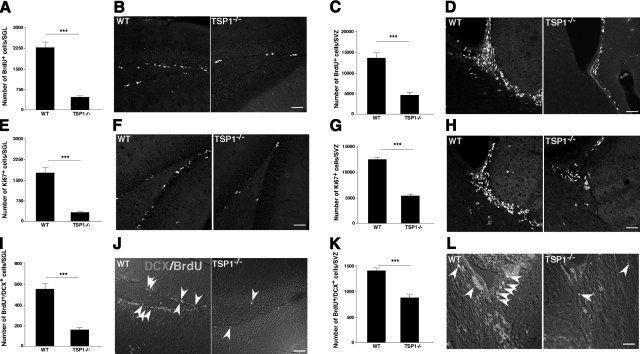
To address a possible role of TSP1 in adult neurogenesis, we first examined the proliferation and neuronal differentiation of neural progenitors in young adult (8–10 wk of age) TSP1−/− mice and in their WT counterparts. Mice were injected with BrdU (50 mg/kg), reinjected after 2 h, and euthanized 24 h later. Brain slices were examined for BrdU incorporation in the SVZ and the SGL. Significantly fewer BrdU+ cells in the SGL were observed in the TSP1−/− mice than in WT controls (Fig. 1A, B). Similar results were obtained for the SVZ (Fig. 1C, D). Both the SVZ and SGL were also labeled with Ki67, an endogenous marker for proliferating cells. Results similar to those with BrdU labeling were obtained (Fig. 1E–H).
Figure 1.
TSP1 deficiency impairs NPC proliferation and neuronal differentiation in vivo. A–H) To examine proliferation of neural progenitors in TSP1−/− and WT mice, exogenous (BrdU; A–D) and endogenous (Ki-67; E–H) proliferation markers were used. Labeled cells were quantified in SGL (A, B, E, F) and SVZ (C, D, G, H) areas of the brain. A significant reduction in proliferating cells was found in both SVZ and SGL of TSP1−/− mice compared to WT counterparts. I–L) To examine neuronal differentiation in TSP1−/− and WT mice, brain slices containing SGL (I, J) and SVZ (K, L) were double-labeled with DCX and BrdU at 7 d after BrdU injection. Significantly less DCX/BrdU double-positive cells were found in TSP1−/− as compared to WT mice. Quantification data are from ≥4 animals/group. Confocal micrographs are representative. ***P < 0.001; Student’s t test. Scale bars = 50 μm (B, D, F, H, L); 20 μm (J).
Next, we examined neuronal differentiation in the SGL and SVZ of TSP1−/− and WT mice. Examination of neuronal differentiation from BrdU-labeled progenitors was performed by double labeling of brain slices for BrdU and doublecortin (DCX) from mice euthanized 7 d after BrdU injection. Significantly fewer DCX/BrdU double-positive cells were found in the SGL (Fig. 1I, J) and SVZ (Fig. 1K, L) of TSP1−/− mice compared to their WT counterparts. Note that the total number of DCX single-positive cells in TSP1−/− mice is substantially reduced compared to WT mice (due to technical limitations to precisely count DCX single-positive cells, quantification data are not provided).
Taken together, these data suggest that NPC proliferation and neuronal differentiation are impaired in both of the neurogenic brain areas of adult TSP1−/− mice. However, these results do not distinguish between intrinsic vs. extrinsic modes of TSP1 action.
We then examined the pattern of TSP1 expression in neonatal vs. young adult (8–10 wk of age) brain using quantitative real-time PCR (qRT-PCR) in two areas, hippocampus and SVZ. The qRT-PCR data demonstrate that TSP1 expression is easily detectable in adult brain, albeit significantly reduced when compared to 7-d-old brains (Supplemental Fig. 1A). The Allen Brain Atlas (ABA; http://www.brain-map.org) provides results for in situ hybridization in the brain of virtually every known gene. On the basis of ABA, TSP1 is indeed expressed throughout the adult brain (Supplemental Fig. 1B, C).
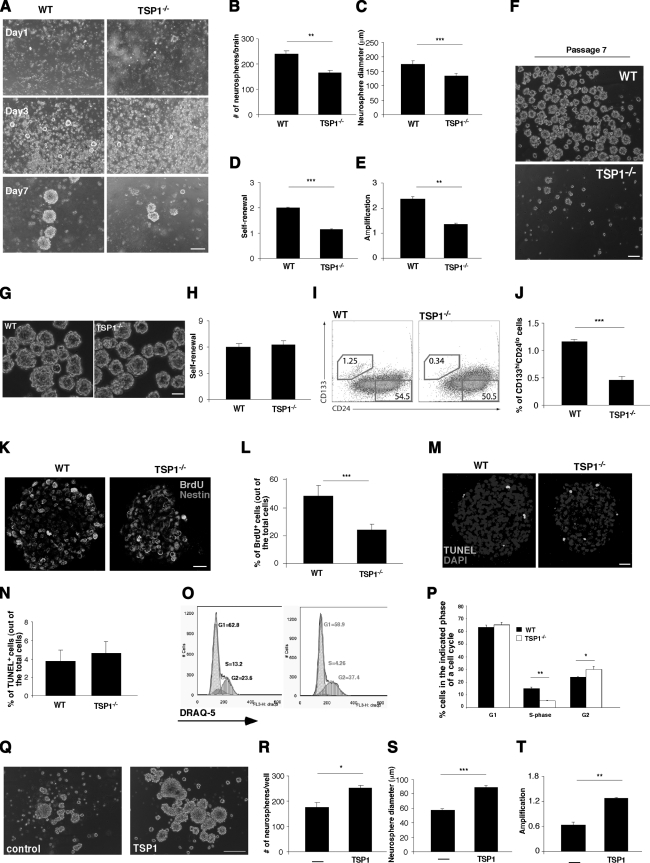
To better understand whether TSP1 contributes to stem cell proliferation as the niche-derived (extrinsic) vs. NPC-derived (intrinsic) factor, we isolated progenitor cells from SVZ of WT and TSP1−/− mice. Primary neurospheres (P1) were formed over the first 7 d in culture. Thereafter, primary neurospheres were dissociated and reseeded as single cells in order to form secondary (P2), then tertiary neurospheres (P3), and so on. Representative micrographs of P1 at different stages of growth are shown in Fig. 2A. Compared to neurospheres generated from WT-derived NPCs, neurospheres generated from TSP1−/−-derived NPCs were significantly fewer in number (Fig. 2B) and smaller in diameter (Fig. 2C), and their capacities for self-renewal (Fig. 2D) and amplification (Fig. 2E) were significantly impaired. Decreased proliferation capacity of TSP1−/− NPCs was exacerbated with every passage in vitro, and by passage 7, NPCs from TSP1−/− mice considerably lagged behind their WT counterparts (Fig. 2F). Before reaching the size of a neurosphere (∼40 μm), they started to adhere and differentiate. Therefore, while NPCs generated from WT mice could be kept in culture for ≥15 passages, TSP1−/−-derived NPCs could not be maintained for >7 or 8 passages in vitro, further suggesting the impaired stemness of TSP1−/−-derived NPCs. Interestingly, however, when NPCs are obtained from neonates (p14), no difference between WT and TSP1−/−-derived neurospheres is evident (Fig. 2G, H). No difference between WT and TSP1−/−-derived neurospheres was evident also when NPCs were obtained from p7 and p21 mice (data not shown). These results suggest that TSP1 is involved in adult neurogenesis but not in neonatal neurodevelopment, which is in line with previously published observations (17).
Figure 2.
TSP1 deficiency impairs NPC proliferation in vitro. A) Phase contrast representative images showing primary neurosphere cultures derived from SVZ of WT and TSP1−/− mice at different time points in vitro. B–E) Quantification of
average number (B), diameter (C), self-renewal (neurospheres/plated cells×100) (D), and amplification (live cells from neurospheres/plated cells) of P1 neurospheres obtained from WT and TSP1−/− mice (E). Data are means ± se from ≥3 independent experiments; ≥4 mice/group. F) TSP1−/− NPC proliferation and sphere formation were terminally impaired at P7, as compared with WT counterparts. G) Phase-contrast representative images showing primary neurosphere cultures derived from SVZ of WT and TSP1−/− p14 pups during P3 in vitro. H) No difference in self-renewal capacity was observed for NPCs obtained from TSP1−/− and WT p14 mice. I) FACS histograms for dissociated WT or TSP1−/− NPCs from P5 labeled for CD133 and CD24. J) Significant reduction in CD133hiCD24lo early progenitor cells is evident in TSP1−/−-derived NPCs. K) To examine proliferation of NPCs, neurospheres from WT and TSP1−/− mice were incubated with BrdU (10 μM) for 8 h and labeled with anti-Nestin (red) and anti-BrdU (green) antibodies. L) Average percentage of BrdU+ cells of total Nestin+ cells from 3 independent experiments; ≥40 neurospheres/group. M) To examine apoptosis, neurospheres from WT and TSP1−/− mice were TUNEL labeled. Micrographs are representative. N) Average percentage of TUNEL+ cells of total cells from ≥3 independent experiments; ≥40 neurospheres/group. O) To determine whether TSP1 affects cell cycle progression, dissociated WT and TSP1−/− NPCs were analyzed by FACS for DNA content using DRAQ-5. Cell cycle histograms are representative. P) Average percentages of G1-, S-, and G2-phase cells from 3 independent experiments. Q–T) P3 neurospheres derived from TSP1−/− mice were treated with 2 μg/ml of purified TSP1 for 72 h. Q) Representative phase-contrast images showing primary neurospheres from TSP1−/− mice at P3 in vitro with and without treatment with purified TSP1. R–T) Quantification of average number (R), diameter (S), and amplification (T) (live cells from neurospheres/plated cells) of TSP1−/− P3 neurospheres treated with purified TSP1 or vehicle. All data are means ± se from ≥3 independent experiments; ≥4 mice/group. * P < 0.05, ** P < 0.01, *** P < 0.001; Student’s t test. Scale bars = 200 μm (A); 100 μm (F, G, Q); 20 μm (K, M).
The impaired proliferation of NPCs from TSP1−/− mice and the lost stemness after 7 passages suggest that the numbers of neural stem cells are reduced in the population of TSP1−/− NPCs. To address this, we labeled single-cell suspensions of neurosphere cultures, during their fifth generation in vitro, for CD133 and CD24 markers (21) and analyzed the subpopulations of progenitor cells by FACS. The subpopulation of neural stem cells, CD133hiCD24lo, was significantly reduced in TSP1−/− cells, as compared to their WT counterparts (Fig. 2I, J).
The percentage of BrdU+ cells, measured in P1 neurospheres that were incubated with BrdU (10 μM) during the last 8 h of the 7-d culture, was lower in TSP1−/−-derived than in WT-derived neurospheres (Fig. 2K, L). This suggests that the impaired self-renewal observed in TSP1−/− P1 cells might be attributable to decreased cell proliferation. To exclude the possibility of accelerated cell death in TSP1−/− neurospheres, P1 neurospheres were labeled for TUNEL-positive (apoptotic) cells. There was no difference in the number of apoptotic cells between WT-derived and TSP1−/−-derived neurospheres (Fig. 2M, N), suggesting that the slow growth observed in NPCs from TSP1−/− mice reflects decreased proliferation of progenitors rather than their accelerated death. Labeling of the cells with DRAQ5 and examination of their cell cycle revealed that the S phase in NPCs from TSP1−/− mice is significantly reduced, whereas their G2 phase is increased (Fig. 2O, P). These results further suggest that proliferation of TSP1−/− NPCs is impaired.
To confirm that TSP1 is required for NPC proliferation, we examined the proliferation of TSP1−/− neurospheres after incubating them for 72 h in the presence of purified TSP1 (2 μg/ml). Representative micrographs are shown in Fig. 2Q. Relative to TSP1−/− neurospheres incubated with vehicle only, the purified TSP1-treated neurospheres were significantly increased both in number (Fig. 2R) and in diameter (Fig. 2S), and their amplification efficiency was substantially increased (Fig. 2T). We also examined the expression of TSP1 by proliferating and differentiating NPC. TSP1 mRNA levels examined by qRT-PCR revealed similar levels of expression in P1, P2, and P3 neurospheres, and a significant induction in its expression on NPC differentiation (Supplemental Fig. 2), suggesting that both proliferating and differentiating progenitor cells can be a source of endogenous TSP1.
Taken together, although the above findings suggest the intrinsic role played by TSP1 in NPC proliferation, the experiments showing that the addition of exogenous purified TSP1 can restore the WT phenotype in TSP1−/− NPCs suggests that niche-derived (extrinsic) TSP1 could also support a proliferating NPC pool.
TSP2 is another member of the thrombospondin family whose role in synaptogenesis has been demonstrated (9). Therefore, we examined proliferation of NPCs obtained from TSP1/2−/− mice. No difference between TSP1−/− and TSP1/2−/− neurospheres was evident for up to 3 generations in vitro (Supplemental Fig. 3A–D). The levels of TSP2 in TSP1−/− neurospheres were not changed, as examined by RT-qPCR (Supplemental Fig. 3E), suggesting that TSP2 does not compensate for the absence of TSP1. These results are further supported by the data from ABA, demonstrating low expression of TSP2 in adult brain (Supplemental Fig. 3F, G). Moreover, in vivo, adult TSP1/2−/− mice did not differ from their TSP1−/− counterparts in the number of proliferating cells and neuronal progenitors in SVZ and SGL (data not shown). These data do not exclude the possible role of TSP2 in the developing brain, which needs to be further investigated.
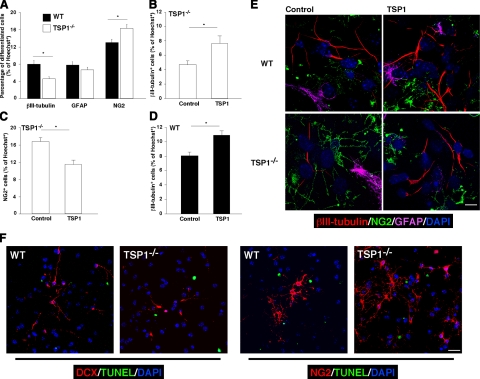
Having observed in vivo (after labeling with DCX; Fig. 1I–L) that neuronal differentiation in TSP1−/− mice is impaired and the increased expression of TSP1 in differentiating neurospheres in vitro (Supplemental Fig. 2), we took a closer look at the possible role of TSP1 in NPC differentiation in vitro. After culturing single-cell suspensions of WT and TSP1−/− NPCs in differentiation medium for 7 d, we immunolabeled them with the neural markers: β-tubulin isotype III (βIII-tubulin; for neurons), GFAP (for astrocytes), and NG2 [for oligodendrocyte precursor cells (OPCs)]. The proportion (mean ± se from ≥4 experiments) of cells labeled with βIII-tubulin in the WT cultures was 8 ± 0.85% compared to only 4.6 ± 0.5% in the TSP1−/− cultures (P<0.05; Fig. 3A). In line with these data, significantly more OPCs were observed in the differentiated TSP1−/− NPCs (16.3±0.9%) than in the corresponding WT (13±0.7%, P<0.05, Fig. 3A). NG2 is often expressed by radial glia progenitors, and NG2+ cells have been shown to differentiate into neurons in vivo(22). However, in vitro, NG2 predominantly labels OPCs (23, 24). Moreover, OPCs in vitro have distinguishable cell morphology; thus, our counts are based on immunolabeling combined with cell morphology examination.
Figure 3.
TSP1 deficiency impairs NPC neuronal differentiation in vitro and could be rescued by purified TSP1. A) P4 NPCs from WT or TSP1−/− mice were differentiated in culture for 7 d and immunolabeled with βIII-tubulin (neurons), GFAP (astrocytes), and NG2 (OPCs). Decreased neurogenesis with increased oligodendrogenesis was observed in NPCs from TSP1−/−, as compared to WT mice. B–D) P4 NPCs from WT or TSP1−/− mice were cultured with or without purified TSP1 (2 μg/ml; every 12 h for 6 d). Immunolabeling for differentiated cells revealed increase in neuronal differentiation (B) and a decrease in oligodendrocyte differentiation (C) after incubation of TSP1−/− NPCs with purified TSP1. D) P4 NPCs from WT mice treated similarly with purified TSP1 exhibited increased neuronal differentiation. Data are means ± se of ≥3 independent experiments. *P < 0.05; Student’s t test. E) Representative micrographs of WT and TSP1−/− NPC differentiation under different conditions. F) P4 NPCs from WT or TSP1−/− mice were differentiated in culture for 7 d and immunolabeled for DCX/TUNEL or NG2/TUNEL to detect neuronal (DCX) and oligodendrocyte (NG2) precursor death. Less than 1% of either DCX+ or NG2+ cells were TUNEL positive; no significant changes were observed between WT and TSP1−/− groups. Scale bars = 30 μm (F); 10 μm (E).
There were no significant differences in GFAP labeling. GFAP, in addition to differentiated astrocytes, also labels radial glia and other subtypes of progenitors (25), which might affect the result interpretation; therefore, we excluded astrocytes from quantification in future experiments and have focused on neuronal differentiation and on OPCs.
To examine whether exogenous purified TSP1 could rescue the capacity for neuronal differentiation of cultured TSP1−/− NPCs, we incubated TSP1−/− NPCs with vehicle or with purified TSP1 (2 μg/ml, added every 12 h for 6 d). Incubation of these cells with purified TSP1 partially restored the neurogenesis (Fig. 3B) and reduced oligodendrogenesis (Fig. 3C).
To test the possibility that TSP1 might serve as a booster for neuronal differentiation, we incubated WT NPCs with purified TSP1. The result was a significant increase in neuronal differentiation compared to that observed in untreated WT NPCs (Fig. 3D). Representative micrographs are presented in Fig. 3E. These results demonstrate that TSP1 is involved in neuronal differentiation, possibly at a point in NPC decision making between neuronal and oligodendrocytic fate commitment.
To examine a possible effect of TSP1 on apoptosis of early-differentiated precursor cells, we differentiated NPCs for 7 d and double-labeled them for either neuronal precursors (DCX+ cells) or OPCs (NG2+ cells) and for TUNEL. No signs of TUNEL-positive DCX+ or NG2+ cells were found (Fig. 3F), suggesting that TSP1 does not affect the precursor cell death.
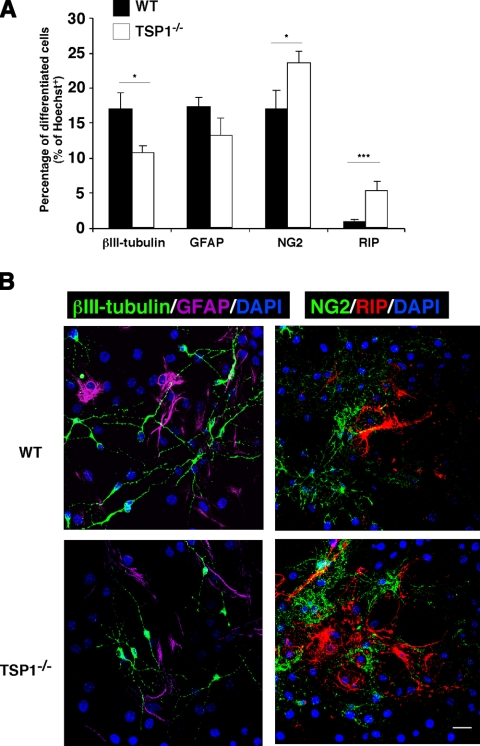
To further address the role of TSP1 on neuronal and oligodendrocyte differentiation, we allowed a longer differentiation of NPCs in vitro (14 d). After 14 d of differentiation, cultures were labeled for βIII-tubulin, GFAP, NG2, and RIP (a marker of differentiated oligodendrocytes). As expected, TSP1−/− NPCs yielded significantly more OPCs (NG2; 23.6±0.6% in TSP1−/−vs. 17±2.6% in WT, mean ± se) and mature oligodendrocytes (RIP; 5.3±1.2% in TSP1−/−vs. 0.9±0.3% in WT), but significantly less neurons (βIII-tubulin; 10.6±1% in TSP1−/−vs. 17±2.3% in WT); no significant difference was obtained for astrocytes (GFAP; 13.1±2.5% in TSP1−/−vs. 17.3±1.2% in WT) (Fig. 4A, B).
Figure 4.
TSP1 deficiency increases mature oligodendrocyte differentiation. P4 NPCs from WT or TSP1−/− mice were differentiated in culture for 14 d and immunolabeled with βIII-tubulin, GFAP, NG2, and RIP (mature oligodendrocytes). Fewer neurons but more OPCs and mature oligodendrocytes were differentiated from TSP1−/− NPCs as compared to WT counterparts. A) Average percentages of βIII-tubulin+, GFAP+, NG2+, and RIP+ cells of total seeded cells were obtained from 4 independent experiments. B) Representative micrographs of differentiated WT and TSP1−/− NPCs immunolabeled for above-mentioned markers. Data are averages ± se. *P < 0.05, ***P < 0.001; Student’s t test. Scale bar = 20 μm.
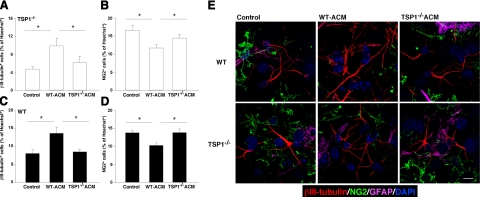
Because purified TSP1 might not be as stable as the endogenous molecule, we repeated these experiments using ACM derived from WT or from TSP1−/− astrocytes (WT-ACM or TSP1−/−-ACM, respectively) as a TSP source. Incubation of TSP1−/−-derived NPCs with WT-ACM increased neuronal differentiation by more than twofold, whereas their incubation with TSP1−/−-ACM had no such effect (Fig. 5A). While WT-ACM decreased oligodendrogenesis (Fig. 5B), TSP1−/−-ACM, however, had no effect on the differentiation of TSP1−/−-derived NPCs.
Figure 5.
TSP1 is required for astrocyte-induced neuronal differentiation. A–D) P4 NPCs from TSP1−/− (A, B) and WT (C, D) mice were cultured for 7 d in the presence of ACM obtained from either TSP1−/− or WT astrocytes. Control cells were kept in regular differentiation medium. Incubation of TSP1−/−-derived NPCs with WT-ACM increased neuronal (A) and decreased oligodendrocyte (B) differentiation. TSP1−/−-ACM did not affect differentiation of TSP1−/− NPCs. Incubation of WT NPCs with WT-ACM resulted in increased neuronal (C) and decreased oligodendrocyte (D) differentiation. TSP1−/−-ACM did not affect differentiation of WT NPCs. E) Representative micrographs of WT and TSP1−/− NPCs differentiated under different conditions.. Data are means ± se of ≥3 independent experiments. *P < 0.05; Student’s t test. Scale bar = 10 μm.
Astrocytes have been shown to promote neuronal differentiation in adult NPCs in vitro(8). We, therefore, examined whether astrocyte-derived TSP1 contributes to this process. Incubation of WT NPCs with WT-ACM significantly increased neurogenesis in vitro (Fig. 5C), as was expected from previous reports (8, 26). Interestingly, however, TSP1−/−-ACM did not promote neuronal differentiation of WT neurospheres (Fig. 5C). TSP1−/−-ACM also did not affect oligodendrogenesis (Fig. 5D). Representative micrographs of WT and TSP1−/− NPCs under different treatment conditions are shown in Fig. 5E. These findings demonstrate that the astrocyte-derived TSP1 molecule plays an important role in astrocyte-mediated neuronal differentiation.
DISCUSSION
In the present study, we demonstrate the role of TSP1 in the maintenance of neural progenitor pool and in neuronal differentiation of NPCs. Depletion of TSP1 in vivo and in vitro resulted in reduced NPC proliferation and impaired neuronal differentiation. Moreover, astrocytes deficient of TSP1 are unable to promote neuronal differentiation from NPCs, suggesting that TSP1 is one of the key astrocyte-derived molecules responsible for astrocyte-mediated neuronal differentiation.
Astrocytes have been previously shown to promote neuronal fate commitment from NPCs through soluble and membrane-bound molecules (8, 27). Our data strongly support that TSP1 is one of the soluble factors produced by astrocytes that mediate neuronal differentiation.
Stem cell renewal and progenitor differentiation are regulated by the specialized microenvironment—the niche—in which these cells reside. These niches are composed of cells (astrocytes are the dominant constituent), soluble and membrane bound molecules, and extracellular matrix. In the adult brain, neural progenitors and their niches are retained in restricted regions, with the local developmental processes occurring throughout the lifetime of the individual. These niches could be seen as islands of neurodevelopment in the finally developed brain. Our results demonstrate that deficiency of TSP1 severely impairs the self-renewal capacity of NPCs both in vivo and in vitro. We have also demonstrated that TSP1 is one of the astrocyte-produced molecules that promote neurogenesis and inhibit oligodendrogenesis from NPCs. Interestingly, a potential anti-TSP1 autoantibody (that also reacts with other molecules) has been reported to promote CNS remyelination (28), further supporting that TSP1 might negatively regulate oligodendrocyte differentiation. It is interesting that TSP1 deficiency did not affect proliferation of NPCs obtained from early postnatal stages (p7, p14, and p21). These results are in line with the previously published observations in vivo, showing no difference in the number of neural progenitors in p17 TSP1−/−vs. WT counterparts (17). These results suggest that TSP1 uniquely contributes to adult neurogenesis and thus could be a clue to better understand the niche requirements for adult neurogenesis as opposed to the developing brain. TSP2, which is another member of the thrombospondin family that has been proposed to benefit synaptogenesis, did not exhibit any effect beyond the effect mediated by TSP1, when NPCs from double-knockout mice (TSP1/2−/−) were examined. Moreover, the TSP family consists of 5 members, one of which (TSP4) is predominantly expressed in mature brain and has been found to be differentially regulated in human vs. nonhuman primates (29). The possible role of other members of this family, and particularly of TSP4, on the maintenance of neural progenitors, their proliferation, and differentiation is of high importance and should be addressed in the future studies.
As an extracellular matrix protein, TSP1 may affect cell fate commitment through interaction with membrane proteins, such as integrin, CD47, CD36, LDL receptor family LRP1, apolipoprotein E receptor 2 (ApoER2), and very low-density lipoprotein receptor (VLDLR), as well as with growth factors such as TGFβ and PDGF. CD47, for example, was demonstrated to promote neural development through activation of Cdc42 and Rac (30). ApoER2 and VLDLR are new ligands for TSP1 (17), and their binding is necessary for postnatal migration of neuronal precursors from the subventricular zone to the olfactory bulb (17). ApoER2 and VLDLR are also the main components of the Reelin signaling pathway, which regulates neuronal migration by influencing Notch signaling (31).
Notch1 signaling is among major pathways that have been shown to influence NPC proliferation and differentiation (32, 33). Activation of Notch1 signaling by Jagged1 or Delta-like1 (34) has been considered as a permissive factor for oligodendrocyte differentiation but against neuronal differentiation (35,36,37). TSP1 may influence Notch signaling by direct binding presumably via interaction of EGF-like motifs, which are present in both TSP1 and Notch components (38), or indirectly through ApoER2 and VLDLR pathway (17).
F3/contactin, another Notch1 ligand that specifically promotes oligodendrocyte differentiation (39), also contains four FN type III-like repeats, which presumably may interact with TSP1 protein (40). Thus, TSP1 might act as a negative regulator of Notch 1 signaling by competitively interacting and interfering with Notch1/Jagged1 and/or Notch1/Contactin binding, as a result of which, neurogenesis is enhanced while oligodendrogenesis is inhibited.
Although the signaling cascade underlying the TSP1-mediated effect on NPC proliferation and differentiation has not been elucidated yet, our data demonstrate that TSP1 is an important regulator of adult neurogenesis and oligodendrogenesis. TSP1 could be a potential therapeutic target for neurodegenerative and demyelinating disorders.
Supplementary Material
Acknowledgments
The authors thank Noel Derecki for assistance with FACS acquisition and data processing. Special thanks are extended to Dr. Bettina Winckler (Department of Neuroscience, University of Virginia) and Dr. Benjamin Purow (Department of Neurology, University of Virginia) for their critical reading and discussion of the manuscript. The authors thank Shirley Smith for editing the manuscript. Dr. Paul Bornstein (University of Washington, Seattle, WA, USA) has kindly provided the original colonies of TSP1−/− and TSP1/2−/− mice. This work was supported, in part, by the Montel Williams MS Foundation grant and, in part, by National Institute of Child Health and Human Development award R21HD056293-01 (to J. K.).
References
- Van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Bjork-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Ziv Y., Ron N., Butovsky O., Landa G., Sudai E., Greenberg N., Cohen H., Kipnis J., Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Leuner B., Mendolia-Loffredo S., Kozorovitskiy Y., Samburg D., Gould E., Shors T. J. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Song H., Stevens C. F., Gage F. H. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Christopherson K. S., Ullian E. M., Stokes C. C., Mullowney C. E., Hell J. W., Agah A., Lawler J., Mosher D. F., Bornstein P., Barres B. A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Adams J. C., Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Agah A., Kyriakides T. R. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Kyriakides T. R., Rojnuckarin P., Reidy M. A., Hankenson K. D., Papayannopoulou T., Kaushansky K., Bornstein P. Megakaryocytes require thrombospondin-2 for normal platelet formation and function. Blood. 2003;101:3915–3923. doi: 10.1182/blood.V101.10.3915. [DOI] [PubMed] [Google Scholar]
- Neugebauer K. M., Emmett C. J., Venstrom K. A., Reichardt L. F. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- O'Shea K. S., Liu L. H., Dixit V. M. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron. 1991;7:231–237. doi: 10.1016/0896-6273(91)90261-w. [DOI] [PubMed] [Google Scholar]
- Osterhout D. J., Frazier W. A., Higgins D. Thrombospondin promotes process outgrowth in neurons from the peripheral and central nervous systems. Dev Biol. 1992;150:256–265. doi: 10.1016/0012-1606(92)90240-h. [DOI] [PubMed] [Google Scholar]
- Blake S. M., Strasser V., Andrade N., Duit S., Hofbauer R., Schneider W. J., Nimpf J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008;27:3069–3080. doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano S., Burns-Cusato M., Atienza M. B., Rahimi D., Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2009;30:483–497. doi: 10.1016/j.neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin T. E., Martens D. J., van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchision D. M., Chen H. L., Pistollato F., Papini D., Ni H. T., Hawley T. S. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- Talbott J. F., Loy D. N., Liu Y., Qiu M. S., Bunge M. B., Rao M. S., Whittemore S. R. Endogenous Nkx22+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Chen X., Zhang C. W., Yang W. L., Wu Y. J., Sun L., Bai L. M., Gu X. S., Ahmed S., Dawe G. S., Xiao Z. C. Morphological and functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of F3/Notch signaling in mouse retina. Stem Cells. 2008;26:580–590. doi: 10.1634/stemcells.2007-0106. [DOI] [PubMed] [Google Scholar]
- Baracskay K. L., Kidd G. J., Miller R. H., Trapp B. D. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55:1001–1010. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- Liour S. S., Yu R. K. Differentiation of radial glia-like cells from embryonic stem cells. Glia. 2003;42:109–117. doi: 10.1002/glia.10202. [DOI] [PubMed] [Google Scholar]
- Barkho B. Z., Song H., Aimone J. B., Smrt R. D., Kuwabara T., Nakashima K., Gage F. H., Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie D. C., Colamarino S. A., Song H. J., Desire L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R., Gage F. H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Asakura K., Pogulis R. J., Pease L. R., Rodriguez M. A monoclonal autoantibody which promotes central nervous system remyelination is highly polyreactive to multiple known and novel antigens. J Neuroimmunol. 1996;65:11–19. doi: 10.1016/0165-5728(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Caceres M., Suwyn C., Maddox M., Thomas J. W., Preuss T. M. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–2321. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- Murata T., Ohnishi H., Okazawa H., Murata Y., Kusakari S., Hayashi Y., Miyashita M., Itoh H., Oldenborg P. A., Furuya N., Matozaki T. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26:12397–12407. doi: 10.1523/JNEUROSCI.3981-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K., Torii M., Sarkisian M. R., Bartley C. M., Shen J., Radtke F., Gridley T., Sestan N., Rakic P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig J. J., Silbereis J., Vaccarino F. M., Sestan N., Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy M. F., Genoud S., Li W. W., Leone D. P., Mantei N., Suter U., Franklin R. J. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–1941. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M. D., Lake R. J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Park H. C., Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development. 2003;130:3747–3755. doi: 10.1242/dev.00576. [DOI] [PubMed] [Google Scholar]
- Genoud S., Lappe-Siefke C., Goebbels S., Radtke F., Aguet M., Scherer S. S., Suter U., Nave K. A., Mantei N. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–718. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Perez S. E., Qiao Z., Verdi J. M., Hicks C., Weinmaster G., Anderson D. J. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Cordle J., Johnson S., Tay J. Z., Roversi P., Wilkin M. B., de Madrid B. H., Shimizu H., Jensen S., Whiteman P., Jin B., Redfield C., Baron M., Lea S. M., Handford P. A. A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nat Struct Mol Biol. 2008;15:849–857. doi: 10.1038/nsmb.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. D., Ang B. T., Karsak M., Hu W. P., Cui X. Y., Duka T., Takeda Y., Chia W., Sankar N., Ng Y. K., Ling E. A., Maciag T., Small D., Trifonova R., Kopan R., Okano H., Nakafuku M., Chiba S., Hirai H., Aster J. C., Schachner M., Pallen C. J., Watanabe K., Xiao Z. C. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C., Gauthier F., Denisenko-Nehrbass N., Le Bivic A., Rougon G., Girault J. A. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr) J Cell Biol. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.