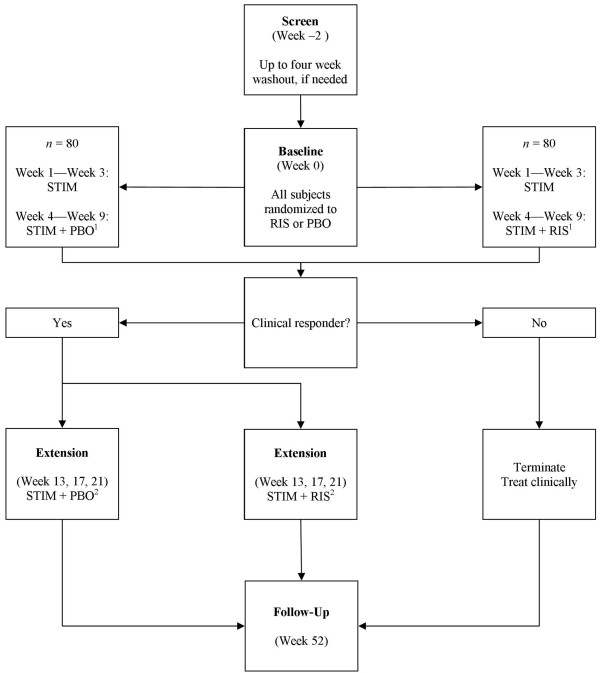
Figure 1.
At screen, arrangements were made to discontinue most medicines for 2 weeks. Fluoxetine and antipsychotics were washed out for 4 weeks. At baseline, participants were randomized to psychostimulant (STIM; usually OROS methylphenidate) plus placebo or combined (STIM + risperidone [RIS]) treatment. 1If subjects did not demonstrate sufficient improvement by end of Week 3 (defined as normative value + 0.5 standard deviation on the NCBRF D-Total score), PBO/RIS was added to treatment. At end of Week 9, subjects were classified as clinical responders (CGI-I = 1 or 2 and NCBRF Disruptive Total reduced by 25% relative to baseline). 2Responders were followed on their originally assigned conditions for 12 weeks of double-blinded Extension. Nonresponders were treated clinically as appropriate, based on the study team's best judgment. On the one-year anniversary date from baseline, all participants were asked to return for a follow-up assessment.