Table 3.
Endogenous lipids, fatty acids and prostaglandin endoperoxide analogs.
| Number | Compound | Structure | IC50[μM]† | Ref. |
|---|---|---|---|---|
| 1 | LTC4 |
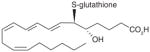
|
5 | [26] |
| 2 | 15-deoxy-Δ12,14-PGJ2 |
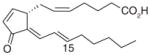
|
0.3 | [73] |
| 3 | U-51605 (PGH2 stable analog) |
|
<10 >100 |
[73] [26] |
| 4 | U-44069 (PGH2 stable analog) |
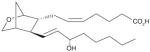
|
NI | [26] |
| 5 | U-46619 (PGH2 stable analog) |
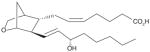
|
NI | [26] |
| 6 | Arachidonic acid |
|
0.3 | [73] |
| 7 | Docosahexaenoic acid |
|
0.3 | [73] |
| 8 | Eicosapentaenoic acid |
|
0.3 | [73] |
| 9 | Palmitic acid |
|
2 | [73] |
Determined by cell-free mPGES-1 activity assays.
NI: No significant inhibition; PGH2: Prostaglandin endoperoxide.
