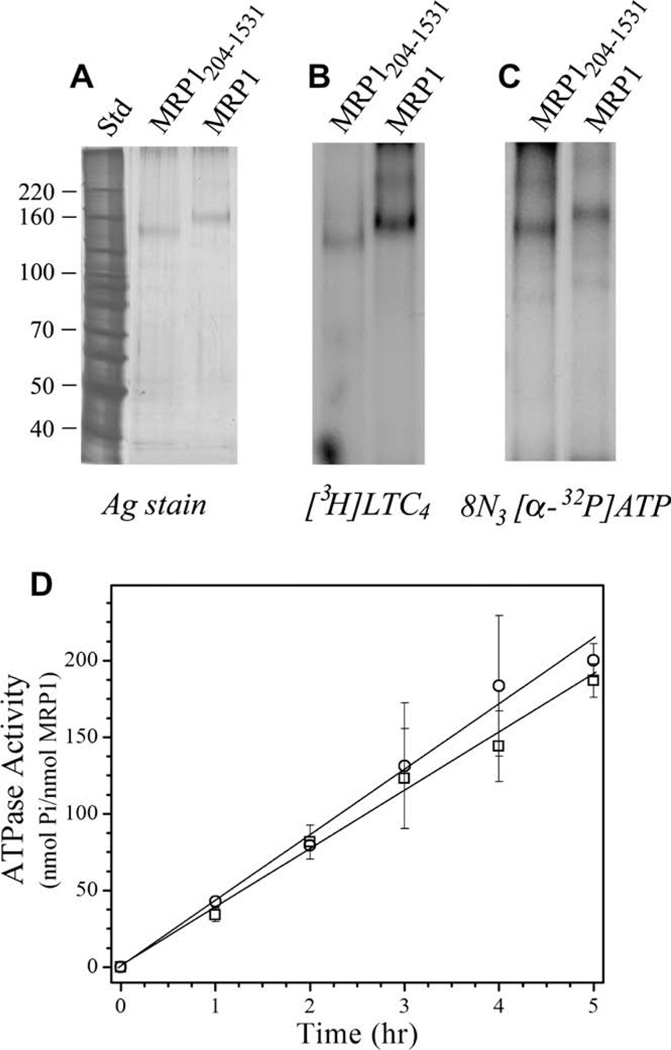
Figure 1.
Properties of purified recombinant MRP1 and MRP1204–1531. expressed in P. pastoris. (A) Purified MRP1 (35 ng) and ‘short’ MRP1204–1531 (28 ng) were resolved by SDS–PAGE and silver stained. Molecular weight markers are shown on the left. (B) Purified MRP1 (1.4 µg) and MRP1204–1531 (0.8 µg) were incubated with 8-azido[α-32P]ATP (2.3 µCi; 5 µM), irradiated at 302 nm, and then resolved by SDS–PAGE and processed for autoradiography. (C) Purified MRP1 (1.5 µg) and MRP1204–1531 (0.9 µg) were incubated with [3H]LTC4 (200 nM; 0.13 µCi), irradiated at 302 nm, and then resolved by SDS–PAGE and processed for autoradiography. (D) MRP1 (0.49 µg) (○) and MRP1204–1531 (0.42 µg) (□) were assayed for ATPase activity over a 5 h time period. Values obtained were corrected for ATP hydrolysis in the absence of protein. Each point represents the mean (±SD) of four determinations.