Abstract
Background and the purpose of the study
It has been well established that cholinergic pathway plays an important role in learning and memory processes. The present study was designed to evaluate the effects of Morris water maze (MWM) training on spatial memory acquisition and expression of the vesicular acetylcholine transporter (VAChT) in male rats.
Methods
In this study, training trials of all groups of animals were conducted in the MWM task. Rats received one training session consisting of four trials per day which continued for another four consecutive days. Controls received visible platform MWM training. The escape latency, the traveled distance and swimming speed for each rat were recorded and used to evaluate the performance of the animal during training period. For evaluation of expression of VAChT protein levels, brain tissues from animals in each experiment were obtained immediately after the last trial on the related experimental day and processed for immunohistochemistry staining and western blotting analysis.
Results
There was a significant difference between animals subjected to one day training and those receiving four days of training in escape latency and travel distance. There were an apparent increase in VAChT immunoreactivity in the medial septal area (MSA) and CA1 region of the hippocampus in one day and four day trained animals compared with controls (visible group). Quantitative immunostaining analysis by optical density measurements in the CA1 region and evaluation of immunopositive neurons in medial septal area of brain sections confirmed qualitative findings. Assessment of VAChT protein level expression in hippocampus by western blotting evaluation showed the same pattern of immunohistochemistry results.
Conclusion
Overall, results of this study reveal changes in cholinergic neuron activity in different stages of training in the MWM task. Data suggest that there is a significant level of cholinergic neuronal activity during early stages of the training especially in the hippocampus region that may contribute to the apparent increase in VAChT expression.
Keywords: Acquisition phase, Cholinergic markers, Hippocampus, Medial septal area, Immunohistochenistry, Western blottig
INTRODUCTION
Cholinergic systems of the mammalian brain play an important role during learning and memory. Acetylcholine (Ach) is one of the key chemical messengers in the brain found at significantly higher concentrations in the rat cortex than classical monoamine transmitters. The degeneration of the forebrain cholinergic projection system is correlated with a deficit in cognitive performance which is associated with aging and the senile dementia of Alzheimer's disease (1, 2). This observation suggests that, at least in part, the cognitive impairment is due to a cholinergic damage. Similarly, experiments with lesions of the forebrain cholinergic neurons made by different neurotoxins allowed pinpointing the role of cholinergic activity in learning and memory (3, 4). A majority of experimental studies have shown that lesions of forebrain cholinergic pathways or the pharmacological blockade of cholinergic transmission cause an impairment of learning and memory as assessed by different memory tasks. Such data support the idea that these systems are critical in memory formation (5, 6).
The vesicular acetylcholine transporter (VAChT) represents a well-known cholinergic marker protein (7). In cholinergic pre-synaptic terminals, ACh is stored by VAChT into synaptic vesicles for regulated exocytosis (8, 9). Furthermore, there is evidence that expression of VAChT can be used to visualize cholinergic neurons in the brain (10). Spatial learning and particularly Morris Water Maze (MWM) performance appear to depend upon the coordinated action of different brain regions and neurotransmitter systems, constituting a functionally integrated neural network. The importance of the cholinergic system for spatial learning and memory has been well-established in studies using the MWM (11–14). It has been shown that decreased expression of vesicular acetylcholine transporter induced learning deficit in rodents (15). In the present study, the effects of training in MWM task were investigated on spatial memory acquisition as well as on VAChT protein expression in the medial septal area and CA1 region of the hippocampus by qualitative and quantitative means. The effects on VAChT expression were analyzed after one day and four days of training.
MATERIALS AND METHODS
Animals
Male Albino-Wistar rats (200–250g) were obtained from Pasteur lnstitute of Iran and housed in groups of five in stainless steel cages, handled daily, and provided food and water ad libitum. A 12-hrs light/12-hrs dark cycle was maintained, and animals were trained during the light cycle. For both behavioral and molecular studies, animals were randomly divided to 3 groups of one day trained, four days trained and control (visible) groups. These animals’ experiments were carried out according to the Ethical Committee for the use and care of laboratory animals of Tehran University of Medical Sciences. All efforts were made to minimize animal suffering.
Behavioral training
In this study, four days training trials of all groups of animals were conducted in the MWM task as described previously (13). A video camera was mounted directly above the water maze pool and the versatile tracking system of EthoVision (Noldus Information Technology, Wageningen, Netherlands) was employed (16) to evaluate the escape latency (the time to reach the hidden platform), traveled distance (the length of swim path), and swimming speed of each rat in training period. In the control group visual experiments were performed by extending a flag above the water level from the submerged platform which makes the platform visible for the animals and was located it in a quadrant.
Immunohistochemsitry and quantification of VAChT immunopositive neurons
Brain tissues from animals in each experiment (one day trained, four days trained and control animals) were obtained immediately after the last trial on the related experimental day and processed for immunohistochemistry staining. Animals were anesthetized and then transcardially perfused with cold PBS followed by 4% paraformaldehyde in phosphate buffer containing 0.15% picric acid. The brains then post-fixed in the paraformaldehyde/picric acid solution followed by incubation in a solution containing 30% sucrose. After being embedded in optimal cutting temperature (OCT), the brains were sectioned at 40 µm intervals at −30°C. These tissue sections of CA1 region of the hippocampus and medial septal area were immunostained for VAChT protein (9) and assessed by BX51 Olympus light microscope using digitized Olysia software (Olympus, Japan). Finally, the images of CA1 region were analyzed for optical intensity of pixel (total intensity/area) by Scion image (Scion Cooperation, Japan) and the number of VAChT-containing neurons in medial septal area (MSA) was determined by counting the cells in the collected brain slices according to the standard protocols (12, 14). All photomicrographs were taken at 10X.
Western blotting
The brains were dissected and bilateral hippo-campuses were removed in the cold artificial cerebrospinal fluid after completion of training process. Hippocampal tissues were minced and homogenized with 200 µl of lyses buffer (10 mM Tris pH of 8, 0.1% SDS, 0.5 mM DTT, 1% NP40, 0.5% Na-deoxycolate, 0.5 mM PMSF, 5 µg/ml leupeptin) at 4°C for 30 min. The samples were centrifuged at 14,000 rpm and 4°C for 5 min, and the supernatant was taken out. Twenty µl of loading buffer (50 mM Tris pH=6.8, 2% SDS, 4% 2-mercaptoethanol, 0.1% bromophenol blue, 10% glycerol) was added to the supernatant and boiled for 8 min. Sixty µl of this product were loaded onto 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoretically separated proteins were transferred to a polyvinylidene fluride membrane (Roche, Germany). Membranes were immunoblotted with anti-VAChT (1:1000) (Santa Cruz, USA) overnight at 4°C, and then incubated with horseradish peroxidase-conjugated anti-goat (1:10,000) antibodies at room temperature for 1 h. Detection was carried out using a chemiluminescence western blotting kit (mouse/rabbit) (Roche, Mannheim, Germany) on BioMax film (Kodak, Rochester, NY).
Immunoreactive bands were digitized using image J 1.410 (NIH, USA) and normalized to β-actin.
Statistics
One-way analysis of variance (ANOVA) was used for comparison of behavioral criteria during training for the one day and four days trained animals. A Newman-Keuls multiple comparison post- test was performed to assess differences between groups. A P-value of 0.05 or less was considered statistically significant.
RESULTS
Evaluation of escape latency, traveled distance, and swimming speed during the training days in the MWM
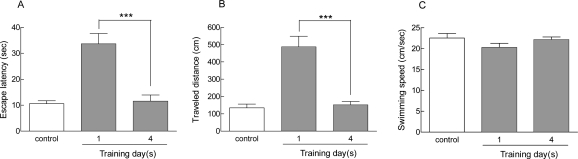
Results from the MWM training trials are summarized in figure 1. After four days training in this task (each day consisted of one block, and each block included four trials), animals learned to find the hidden platform. This was indicated by a significant decrease in escape latency and traveled distance compared to those of the one day trained animals (Fig 1A and B). There was a significant difference (***p<0.001) between the fourth and first day of training in terms of escape latency and traveled distance for finding the hidden platform. Statistical analysis also showed a significant difference between the control (visible) group and the one day trained animals, but there was no significant difference between the four days trained animals and the visually guided platform-tested animals. The swimming speed was not significantly affected by training days, indicating no motor disturbances in any group of animals (Fig 1C).
Figure 1.
Effect of one day and four days of training on spatial memory. Results show that all animals learned to find the hidden platform during the training days. There was a significant decrease (***p<0.001) in escape latency (A) and traveled distance (B) on the fourth day of training compared to the first day. Swimming speed did not change significantly in any group of animals (C). Values are presented as means±SEM for at least 7 animals per group.
Evaluation of VAChT expression in dorsal hippocampus by immunohistochemistry and western blot analysis
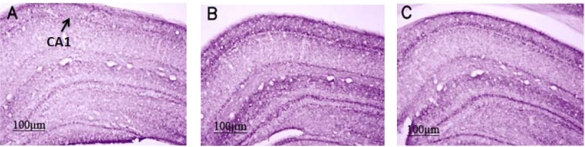
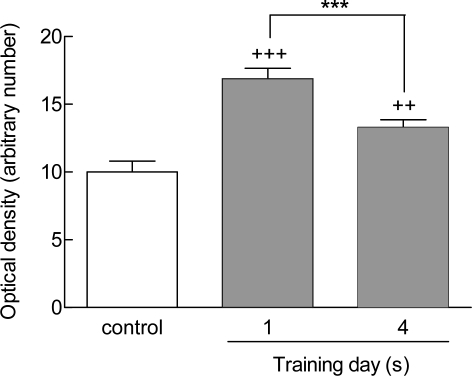
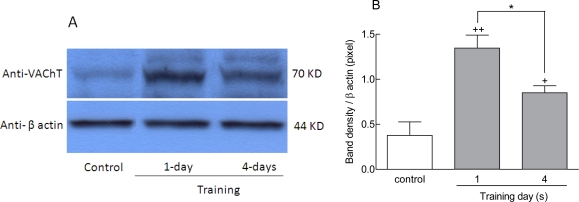
Sections of rat brain tissue from controls, one day, and four days trained animals were immunostained with anti-VAChT antibodies. A qualitative increase was observed in the density of VAChT staining in the CA1 region for the one day and the four days trained animals (Fig 2B and C) in comparison to the control group (Figur 2 A). Also, quantitative analysis showed a significant increment in the optical density of VAChT staining in the hippocampus (Fig 3). The staining level in the CA1 region increased in one day trained animals compared to control group (+++p<0.001) (Fig 3). There was additionally an increase in the staining level within the CA1 region in four days-trained animals compared to the control group (++p<0.01) (Fig 3). The extent of staining decreased in four days-traiend animals compared to one day trained animals (p<0.001) (Fig 3). The same pattern of VAChT expression was also observed in western blot analysis (Fig 4). These findings indicated that the expression of protein levels of hippocampal VAChT increased significantly after one (++p<0.01) and four days (+p<0.05) of training quantitatively (Figs 4 A and B). There was also a significant difference in VAChT expression between first and fourth day of training (*p<0.05, Figs 4 A and B).
Figure 2.
Effect of training on the density of VAChT-staining in the dorsal hippocampus. There was an apparent increase in the density of VAChT immunoreactivity of CA1 region in animals that were trained for one (B) and four days (C) compared to the control group (A). Scale bar=100 µm.
Figure 3.
VAChT staining intensity in the CA1 region of one-day and four days trained animals. Immunoreactivity levels were significantly higher in one day-trained rats compared to controls (+++p<0.001). Staining levels in the CA1 region were also elevated in four days trained animals in comparison to controls (++p<0.01) but were lower compared to one day trained animals (***p<0.001). Data was presented as means±SEM of 7 animals per group.
Figure 4.
Evaluation of hippocampal VAChT expression in different stage of training period. Expression of VAChT protein in dorsal hippocampus was significantly increased after one day (++p<0.01) and four days (+p<0.05) of training in comparison to control animals (A and B). Columns represent means±SEM of 7 animals per group.
Effects of training on cholinergic cells in the medial septal nucleus
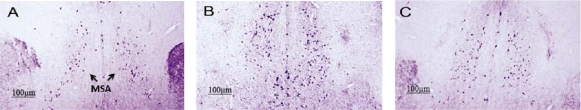
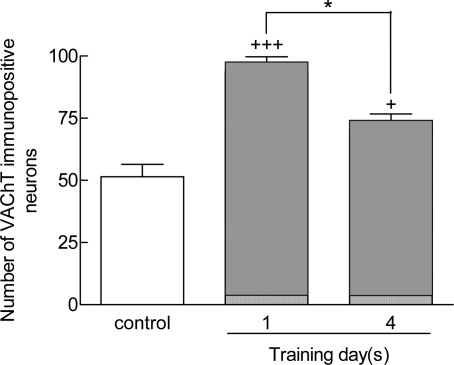
In the MSA, the number of VAChT-immunopositive neurons was elevated with training process (Fig 5 B and C) compared to control sections (Fig 5A). The number of VAChT-stained neurons was higher in one (+++p<0.001) and four days trained animals (+p<0.05) compared to the control group (Fig 6). The number of stained neurons in four days trained animals was lower in comparison to one day trained rats (*p<0.05) (6).
Figure 5.
VAChT-immunoreactivity in the MSA in the control group (A), one (B), and four days trained (C) animals. Staining was more intense in one day trained (B) and four days trained (C) animals compared to the control animals (Panel A). Scale bar=100 µm.
Figure 6.
Number of VAChT-stained neurons in one day trained and four days trained animals. VAChT-positive cells are more frequent in one daytrained animals (+++p<0.001 ) and four days trained animals (+p<0.05) compared to controls. VAChT-immunoreactive neurons declined after four days training. Data represent as means±SEM of 7 animals in each group.
DISCUSSION
There is abundant evidence that the hippocampus is critically involved in learning and memory in general and spatial learning in particular (17). Furthermore, the role of the cholinergic system in spatial learning has been well-established, and Ach antagonists have accordingly been shown to block these functions. Such deficits are probably the result of direct effects on the cholinergic system and not of the secondary side effects on other neurochemical transmitter systems (17, 18).
The results of the present study clearly provide evidence that rats, during a four days training period, progressively learn to find the hidden platform in the MWM test system. This is indicated by the observed and significant reduction of escape latency and traveled distance in the four day trained compared with one day trained animals. Data of this study also reveal that the MWM performance task appears to be most likely completed and established after four days of training. The training did not cause any significant difference in the swimming speed, indicating that the animals’ motor, motivational, and visual abilities did not change during the four days of training. Besides behavioral performance, the expression of VAChT in the CA1 region of the hippocampus and in the MSA after one day training, when the learning process is not yet fully engraved, and after four days training, when learning is presumably fully established was evaluated. Immunohistochemical and western blot findings showed that training increase the number and staining intensity of the VAChT-containing neurons in the CA1 region and the MSA in one day and four days trained animals qualitatively and quantitatively in comparison with the control group. Interestingly, this effect was more prominent in one day than in four days trained rats. Cholinergic input to the hippocampus from the basal forebrain has been ascribed as particularly important for learning and memory processes (1, 18). The basal forebrain is formed by closely associated nuclei- containing medial septal area (MSA) that project cholinergic neurons to the hippocampus predominantly. The importance of MSA in learning process is based on the observation that selective lesions of the medial septum which reduce the activity of ACh-containing neurons impair memory performance assessed by different memory tests (19–21). Such results suggest that the activation of the septohippocampal cholinergic system is important for learning and memory functions. Therefore, the apparent increases in the expression of VAChT in the CA1 region and the MSA following the water maze task, observed in this study, suggest and confirm activation of the septohippocampal cholinergic system during MWM performance and its significant role in spatial learning tasks.
Findings of this study show that VAChT expression after one day training is qualitatively and quantitatively higher than after four days of training. Based on this observation, it might be speculated that cholinergic activation is more intense during early stages of the acquisition phase of learning. Indeed, other reportshave described that cholinergic activation is involved in the acquisition phase (15). For instance, the data indicate that extensive lesions, involving more than 90% of cholinergic neurons, result in the impairment of MWM acquisition (22). In addition, microdialysis experiments depict that an increase in cortical Ach release during performance of simple operant tasks is limited to early acquisition stages (23). Similarly, it has been reported that there were large increases in cortical and hippocampal Ach release during acquisition of a rewarded operant behavior but not during its recall 24.
Activation of Ach receptors and the protein kinase C (PKC) pathway also appear to be implicated in the acquisition phase of learning. PKC trafficking behaves similarly during early phases of acquisition of spatial learning task in the hippocampus but not during later phases 25–27. Therefore, behavioral data and the apparent increase in VAChT expression of this study suggest that cholinergic pathways appear to be more active during the acquisition phase than in the later stages of learning.
In summary, data of this study reveal that the acquisition of spatial learning processes is improved after four days of training in the MWM task. In addition, it a qualitative and quantitative increase in VAChT expression in cholinergic neurons in the dorsal hippocampus and the medial septal area was demonstrated. This increase in VAChT expression was more intense during early rather than the late stages of learning acquisition pointing a selective role in this process.
ACKNOWLEDGEMENT
This work was supported by funds from Tehran University of Medical Sciences and National Center of Excellence of Toxicology.
REFERENCES
- 1.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1987;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 2.Perry EK, Perry RH, Blessed G, Tomlison BE. Necropsy evidence of cerebral cholinergic deficits in senile dementia. Lancet. 1997;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 3.Nieto-Escamez FA, Sanchez-Santed F, De Bruin JP. Cholinergic receptor blockade in prefrontal cortex and lesions of the nucleus basalis: implications for allocentric and egocentric spatial memory in rats. Behav Brain Res. 2002;134:93–112. doi: 10.1016/s0166-4328(01)00458-2. [DOI] [PubMed] [Google Scholar]
- 4.Ridley RM, Baker HF, Leow-Dkye A, Cummings RM. Further analysis of the effects of immunotoxic lesions of the basal nucleus of Meynert reveals substantial impairment on visual discrimination learning in monkeys. Brain Res Bull. 2005;65:433–442. doi: 10.1016/j.brainresbull.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Smith G. Animal models of Alzheimer's disease: experimental cholinergic denervation. Brain Res. 1988;472:103–118. doi: 10.1016/0165-0173(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 6.Melis F, Stancampiano R, Imperato A, Carta G, Fadda F. Chronic ethanol consumption in rats: correlation between memory performance and hippocampal acetylcholine release in vivo. Neuroscience. 1996;74:155–159. doi: 10.1016/0306-4522(96)00109-1. [DOI] [PubMed] [Google Scholar]
- 7.Parsons SM, Prior C, Marshall I.G. Acetylcholine transport, storage and release. Int Rev Neurobiol. 1993;35:279–393. doi: 10.1016/s0074-7742(08)60572-3. [DOI] [PubMed] [Google Scholar]
- 8.Bejanin S, Cervini R, Mallet J, Berrard S. A unique gene organization for two cholinergic markers, choline acetyltransferase and a putative vesicular transporter of acetylcholine. J Biol Chem. 1994;269:21944–21947. [PubMed] [Google Scholar]
- 9.Roghani A, Shirzadi A, Butcher LL, Edwards RH. Distribution of the vesicular transporter for acetylcholine in the rat central nervous system. Neuroscience. 1998;82:1195–1212. doi: 10.1016/s0306-4522(97)00291-1. [DOI] [PubMed] [Google Scholar]
- 10.Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the Putative Vesicular Acetylcholine Transporter in Rat Brain and Localization in Cholinergic Synaptic Vesicles. J Neurosci. 1996;76(7):2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharifzadeh M, Zamanian A R, Gholizadeh S, h, Tabrizian K, Etminani M, Khalaj S, Zarrindast M.R, Roghani A. Post-training intrahippocampal infusion of nicotine–bucladesine combination causes a synergistic enhancement effect on spatial memory retention in rats. Eur J Pharmcol. 2007;562:212–220. doi: 10.1016/j.ejphar.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 12.Karimfar MH, Tabrizian K, Azami K, Hosseini-Sharifabad A, Hosseini A, Pourghorban M, Aghsami M, Gholizadeh S, Abdollahi M, Roghani A, Sharifzadeh M. Time course effects of lithium administration on spatial memory acquisition and cholinergic marker expression in rats. DARU. 2009;17:113–123. [Google Scholar]
- 13.Tabrizian K, Najafi S, Belaran M, Hosseini-Sharifabad A, Azami K, Hosseini A, Soodi M, Kazemi A, Kebriaeezadeh A, Sharifzadeh M. Effect of selective iNOS inhibitor on spatial memory in recovered and non-recovered ketamine induced-anesthesia in wistar rats. IJPR. 2010;15:313–320. [PMC free article] [PubMed] [Google Scholar]
- 14.Azami K, Etminani M, Tabrizian K, Salar F, Belaran M, Hosseini A, Hosseini-Sharifabad A, Sharifzadeh M. The quantitative evaluation of cholinergic markers in spatial memory improvement induced by nicotine-bucladesine combination in rats. Eur J Pharmacol. 2010;636:102–107. doi: 10.1016/j.ejphar.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 15.De Castro BM, Pereira GS, Magalhaes V, Rossato JI, De Jaeger X, Martins-Silva C, Leles B, Lima P, Gomez MV, Gainetdinov RR, Caron MG, Izquierdo I, Cammarota M, Prado VF, Prado MAM. Reduced expression of the vesicular acetylcholine transporter causes learning deficits in mice. Genes. Brain and Behavior. 2009;8:23–35. doi: 10.1111/j.1601-183X.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 16.D’ Hooge R, De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 17.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1998;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 18.Kesner RP. Reevaluation of the contribution of the basal forebrain cholinergic system to memory. Neurobiol Aging. 1988;9:609–616. doi: 10.1016/s0197-4580(88)80122-2. [DOI] [PubMed] [Google Scholar]
- 19.Whishaw IQ, O'Connor WT, Dunnett SB. Disruption of central cholinergic systems in the rat by basal forebrain lesions or atropine: effects on feeding, sensorimotor behaviour, locomotor activity and spatial navigation. Behav Brain Res. 1985;17:103–115. doi: 10.1016/0166-4328(85)90023-3. [DOI] [PubMed] [Google Scholar]
- 20.Rawlins J.N, Olton D.S. The septo-hippocampal system and cognitive mapping. Behav Brain Res. 1982;5:331–358. doi: 10.1016/0166-4328(82)90039-0. [DOI] [PubMed] [Google Scholar]
- 21.Flicker C, Dean RL, Watkins DL, Fisher SK, Bartus RT. Behavioral and neurochemical effects following neurotoxic lesions of a major cholinergic input to the cerebral cortex in the rat. Pharmacol Biochem Behav. 1983;18:973–981. doi: 10.1016/s0091-3057(83)80023-9. [DOI] [PubMed] [Google Scholar]
- 22.Leanza G, Nilsson OG, Wiley R.G, Bjorklund A. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: Behavioral, biochemical and stereological studies in the rat. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 23.Muir JL. Attention and stimulus processing in the rat. Cogn Brain Res. 1996;3:215–225. doi: 10.1016/0926-6410(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 24.Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- 25.Bank B, De Weer A, Kuzirian AM, Rasmussen H, Alkon DL. Classical conditioning induces long-term translocation: of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci USA. 1988;85:1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo PJ, Wetse WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci USA. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douma BR, Van der Zee EA, Luiten PG. Translocation of protein kinase Cgamma occurs during the early phase of acquisition of food reward spatial learning. Behav Neurosci. 1998;112:496–501. doi: 10.1037//0735-7044.112.3.496. [DOI] [PubMed] [Google Scholar]