Table 1. X-ray data-collection and processing statistics for the native wild-type α1-antitrypsin crystal structure 3ne4 .
Values in parentheses are for the highest resolution shell.
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 114.4, b = 38.9, c = 88.8, β = 104.3 |
| Resolution (Å) | 42.11–1.81 (1.91–1.81) |
| No. of reflections | |
| Total | 92961 |
| Unique | 34169 |
| Rmerge† | 0.07 (0.274) |
| Completeness (%) | 98.5 (99.1) |
| Multiplicity | 2.7 (2.6) |
| 〈I/σ(I)〉 | 10.0 (3.4) |
| Rcryst‡ (%) | 18.7 |
| Rfree§ (%) | 23.3 |
| Bave¶ (Å2) | |
| Main chain | 23.9 |
| Side chain | 28.8 |
| No. of water molecules | 217 |
| Ramachandran plot, residues in (%) | |
| Preferred region | 96.5 |
| Allowed region | 3.3 |
| Disallowed region | 0.3 |
| R.m.s.d. from ideal | |
| Bond lengths (Å) | 0.015 |
| Bond angles (°) | 1.5 |
R
merge = 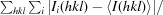
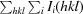 , where i are the set of observations for each reflection hkl.
, where i are the set of observations for each reflection hkl.
R
cryst = 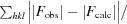
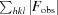 .
.
R free = R cryst for 5% of reflections omitted from refinement.
B ave values are average temperature factors for all molecules in the asymmetric unit.
