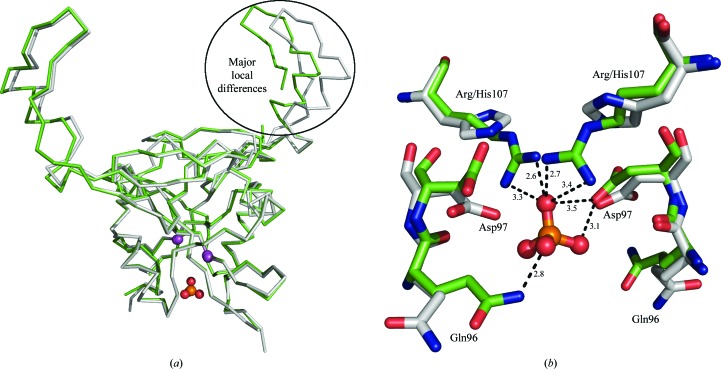
Figure 2.
Structural alignment of dimers of H107R-variant (green) and wild-type (grey) mNKR-P1A. (a) Chains are represented by Cα traces, the Cα atoms of Arg/His107 are shown as magenta spheres and the additional phosphate ion is shown in ball-and-stick representation. The region of major local differences, the extended loop (chain B in the H107R variant), is highlighted by an oval. (b) A detailed view of the mutation-induced asymmetric binding of the phosphate ion compared with the wild type. Amino acids are represented by sticks and the phosphate ion is shown in ball-and-stick representation; the hydrogen-bonding network with distances in Å is only displayed for the discussed phosphate ion.